Molecular Playground/IntegrinBeta1
From Proteopedia
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground. Molecular Playground banner: "
Banner: Studying integrin-mediated metastasis in vitro
|
Contents |
Structure
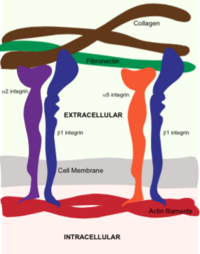
Integrins are surface proteins that are comprised of an alpha subunit and a beta subunit to form a functional transmembrane structure. The subunits of the heterodimer each have small cytoplasmic domains for signal transduction within the cell. There are 18 alpha and eight beta subunits in mammals. Different pairs of these alpha and beta subunits uniquely bind to extracellular proteins. Integrins connect the extracellular environment to the inside of a cell by inducing signals that affect cell functions.
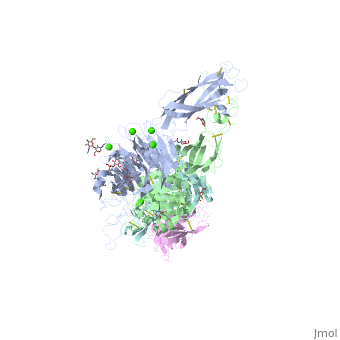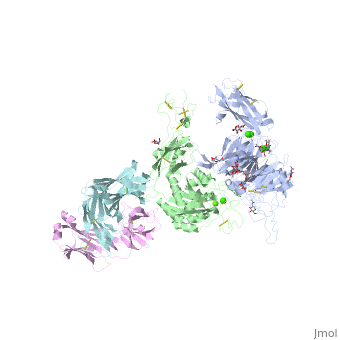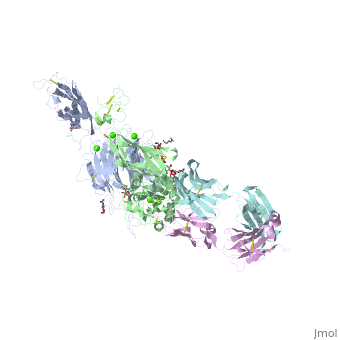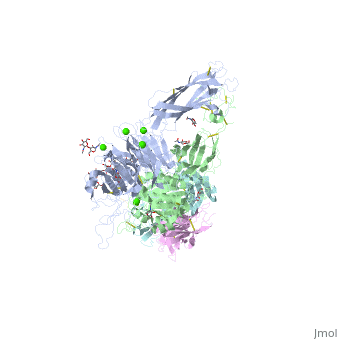
The protein structure on the right has the following color coding: Blue-Alpha 5 chain, Red-Beta 1 chain, Grey-SG/19 Fab fragment light chain, Green-SG/19 Fab fragment heavy chain.
Function
Integrins are a class of surface proteins that bind to extracellular matrix components and transmit chemical and mechanical cues to internal signaling pathways. Integrin beta 1 binds many proteins when dimerized with an alpha subunit, including , laminin and fibronectin. Integrin adhesion to the extra cellular matrix is key for cell ability to adhere, migrate and proliferate in both 2D and 3D systems. These will eventually form adhesion complexes, which regulate actomyocin polymerization. During migration, cells continually form new focal adhesions at the leading edge of the cell and release adhesion complexes at the back of the cell, enabling forward movement.
Here are the N-acetyl-D-glucosamine may regulate integrin signaling during cancer cell migration[1].
Integrins and cancer
Integrins binding to the extracellular matrix provide cells the ability to migrate and remodel the surrounding microenvironment. Integrins are extremely important in cancer metastasis and the progression of solid tumors. Cells bind to extracellular matrix proteins, like, with integrins to stimulate survival, migration/invasion, and proliferation [2]. Though high expression of integrin beta 1 has been shown to drive primary tumor progression and metastasis, targeting integrins for cancer treatment has seen limited clinical efficacy [3]. These failures stem from differential integrin expression between cancer cells, but new technologies to screen cancer cell populations are being developed to affective therapies on a patient-specific level [4]. These technologies will likely allow for more selective treatment and better clinical efficacy for integrin inhibitors.
Studying Integrin Beta-1 in vitro
Due to their roles in both disease and cell-matrix interactions, there is a growing interest in studying integrin function in vitro. Common approaches use simple end point gene or protein expression analysis, however, in vitro studies allow for functional analysis to observe intern binding interactions in single living cells. Tools include siRNA to prevent integrin gene expression, integrin binding peptides (i.e., the RGD peptide) which bind to integrins competitively with ECM proteins)[5], and function-affecting antibodies which can bind to integrin heterodimers and prevent binding to the ECM[6]. When beta1 dimerizes with alpha1, it can bind to both collagens I and IV as well as laminins [7], which are both large components of the ECM of many tissues. Integrin alpha1 beta1 binding to a function affecting integrin antibody is shown These tools are especially useful when microenvironment stiffness[8] or composition[9] are controlled, allowing for examination of the effect of each of these cues on cell phenotype[10] or downstream signaling.
Peyton Lab Research Interests
The Peyton lab studies how cells process chemical and physical cues from the extracellular matrix and how these interactions play a role in the progression of cardiovascular disease and cancer. We aim to understand the downstream signaling pathways activated when cells use their integrins to bind to matrix and how this translates to the disease of interest. We are further interested in how integrins can be biomarkers in disease, because they transduce signals from the diseased microenvironment to cells, likely facilitating disease progression in some cases.
References
- ↑ Saravanan C, Liu FT, Gipson IK, Panjwani N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J Cell Sci. 2009 Oct 15;122(Pt 20):3684-93. doi: 10.1242/jcs.045674. Epub 2009, Sep 15. PMID:19755493 doi:http://dx.doi.org/10.1242/jcs.045674
- ↑ Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010 Jan;10(1):9-22. doi: 10.1038/nrc2748. PMID:20029421 doi:http://dx.doi.org/10.1038/nrc2748
- ↑ dos Santos PB, Zanetti JS, Ribeiro-Silva A, Beltrao EI. Beta 1 integrin predicts survival in breast cancer: a clinicopathological and immunohistochemical study. Diagn Pathol. 2012 Aug 16;7:104. doi: 10.1186/1746-1596-7-104. PMID:22894137 doi:http://dx.doi.org/10.1186/1746-1596-7-104
- ↑ Barney LE, Dandley EC, Jansen LE, Reich NG, Mercurio AM, Peyton SR. A cell-ECM screening method to predict breast cancer metastasis. Integr Biol (Camb). 2014 Dec 24. PMID:25537447 doi:http://dx.doi.org/10.1039/c4ib00218k
- ↑ Matsuno H, Stassen JM, Vermylen J, Deckmyn H. Inhibition of integrin function by a cyclic RGD-containing peptide prevents neointima formation. Circulation. 1994 Nov;90(5):2203-6. PMID:7955174
- ↑ Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997 Apr 7;137(1):231-45. PMID:9105051
- ↑ Karpusas M, Ferrant J, Weinreb PH, Carmillo A, Taylor FR, Garber EA. Crystal structure of the alpha1beta1 integrin I domain in complex with an antibody Fab fragment. J Mol Biol. 2003 Apr 11;327(5):1031-41. PMID:12662928
- ↑ Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2011 Apr;26(4):730-8. doi: 10.1002/jbmr.278. PMID:20939067 doi:http://dx.doi.org/10.1002/jbmr.278
- ↑ Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004 Dec;25(28):5947-54. PMID:15183609 doi:http://dx.doi.org/10.1016/j.biomaterials.2004.01.062
- ↑ Wu P, Hoying JB, Williams SK, Kozikowski BA, Lauffenburger DA. Integrin-binding peptide in solution inhibits or enhances endothelial cell migration, predictably from cell adhesion. Ann Biomed Eng. 1994 Mar-Apr;22(2):144-52. PMID:8074327
Proteopedia Page Contributors and Editors (what is this?)
Lauren Barney, Lauren Jansen, Elizabeth Brooks, Michal Harel, Alyssa Schwartz