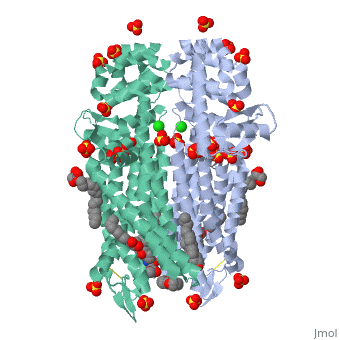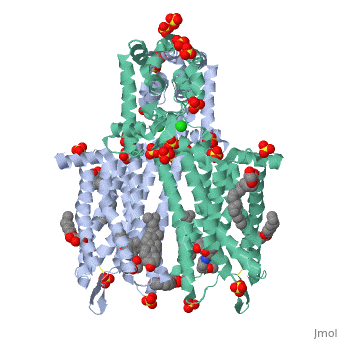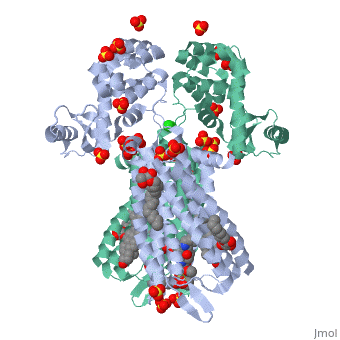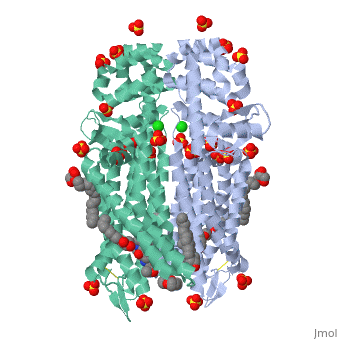
Mu Opioid Receptor Bound to a Morphinan Antagonist
From Proteopedia
Introduction and Background Information
Opium is one of the oldest drugs known to mankind and still has prevalence today. Morphine and codeine, two opiate products, are heavily used both in clinical settings and illegally to to control pain and produce analgesic and unwanted side effects. Mu-opioid receptors are a group of G-protein-coupled receptors and have been found to be the target for the majority of effects of opioid alkaloids.[1] There are 17 known opioid receptors with the three primary types being the μ, κ, and δ. The μ opioid receptor (μ-OR) is the most related to opioid use. There are three subtypes μ1, μ2, μ3 and most is known about μ1.[2] The μ-ORs are part of the neurohormonal system. Within the body (sans drugs) endogenous small molecules naturally produced in the CNS and other glands act upon the GPCRs. Endogenous opioid peptides produce can range in action from pain reduction to regulating diarrhea.[3]
Overview of StructureThe structure of μ-opioid receptor consists of seven transmembrane alpha-helicies (TM1-7) connected through three extracellular loops and three intracellular loops and is 400 amino acids in length. The greater multi-unit μ-opioid receptor has altering aqueous and lipid layers with receptor proteins arranged in parallel dimers. Transmembrane helicies tightly associate while TM1 and TM2 have more limited contact. These strands allow the single chain structures to polymerize. TM3 and ECL2 are connected through a between Cys140 and Cys217. This bridge is conserved across GPCRs as is the seven helix structure. It has been found that a mutation of Thr279 to a lysine group causes the protein to be constituatively active.[4] A possible reason for this could be that the lack of an interaction between that is usually present and may help to stabilize the receptor in its inactivated form.
Ligand Binding Pocket and SpecificityThe μ opioid receptor differs from many other GPCRs. The majority of GPCR have a binding area that is partially buried within the helical region and must get past side chains to bind in the pocket. An example of this can be seen in the M3 muscarinic receptor.[5] The μ opioid receptor exhibits a much shallower and less protected binding pocket. This difference is likely the basis of the varying dissociation kinetics associated with these GPCRs. This structure may also explain why some extremely potent opioids have such rapid dissociation half-lives. This unique feature allows for some drugs such as etorphine to be used as an extremely fast acting analgesic coupled with diprenophine as a reversing reagent. This drug combination is utilized in captive and free-range mammals.[6]
In this crystal structure of the μ opioid receptor it is (β-FNA), a close relative of morphine that is bound in the pocket. When β-FNA binds there are nine residues that have direct interaction with the ligand while there are 14 total within 4Â of β-FNA. The nine residues in direct contact are conserved in the κ and δ opioid receptors. is important because it forms a bond with the amine on the ligand. It is also notable because it is conserved across all opioid receptor subtypes. indirectly interacts with the aromatic ring of the ligand. Rather than bind directly it is thought to employ two water molecules that line up and form a hydrogen bond chain to the hydroxyl group on the morphinan ring. The μ opioid receptor has three distinct differences in the binding domain from the δ receptors. are present in μ-OR which are Asp, Trp and Leu in the δ structure. The Trp318 could be a source of selectivity. When attempting to bind to certain naltirindole, a selective antagonist of the δ receptor, the Trp318 side chain clashes and does not allow for it while the leucine in the same position would accommodate this structure.[7] Small peptides named endomorphins 1 and 2 were shown to have the highest selectivity profile for the µ-OR. Endomorphin 1 shows a 15,000 fold increase in selectivity for µ-OR over κ-OR.[8] Such a high selectivity is a property that is being examined and looked at for the possibility of certain therapies. Better understanding the manner in which an edogenous peptides bind could be clinically useful in addiction studies. Little is known about the manner in which they bind but it is clear that there is a large difference between different opioid receptors.[8] Oligomeric PropertiesAs discussed the μ-OR has the ability to associate and pair with other μ-ORs to form and likely larger groupings. Biological research has suggested that it is likely that the receptors aggregate together and the crystal structure reinforces this.[9] Oligomers have been observed in other GPCRs which gives further support of this possibility.[10] The linking of multiple units is of interest because μ-OR as part of a homo or heterodimer or oligomer could affect morphine tolerance. Its possible that DAMGO and methadone (both agonists) could reduce tolerance to morphine through endocytosis by the μ-OR oligomer. [11] Expressing multiple forms of opioid receptors showed profiles that were different from either expressed alone.[12] More support for oligomerization as a direct function of the μ opioid receptors is the amino acid dimer interface had an extremely high degree of homology with the δ-OR.
Other Aspects and Addictionμ-OR has been shown to be a great target for controlling pain. However such benefits do not come without side effects and drawbacks. The μ-OR is currently at the forefront of research in many addiction diseases as well as therapies.[13] One study found that, addiction from non opioid drugs such as alcohol, cannabinoids, and nicotine are heavily dependent on the μ-OR (all of these act at a different receptor). In μ-OR deficient mutant mice addictions typically seen from these compounds were strongly diminished. This highlighted the possibility of the μ-OR as a convergent molecular switch that can act through direct or indirect activation.[13] Furthermore, it is possible that slight mutations in the µ-OR could be linked to addiction. In one study it was found that in 10% of the former heroine addict population they studied there was a variant receptor that contained a A118G SNP. This SNP causes an Asn40 to Asp40 amino acid substitution. Although this variant receptor has not been shown to have altered affinities to most opioid peptides, it did have a binding affinity approximately three times that of the normal receptor for β-endorphin, an endogenous opioid. β-endorphin is also three times more potent when bound to the receptor and will be important in the future for understanding addictive diseases.[14]
ConclusionThe μ-OR crystal structure elucidated in "Crystal structure of the µ-opioid receptor bound to a morphinan antagonist" is the first high resolution receptor of its kind. Although opiates have been used for centuries current opioid receptor agonist drugs are far from perfect. The new understanding of the structure will enable drug discovery programs to take a structure based approach to finding new drugs and possibly treating some of the underlying causes of addiction. Such a drug could prove helpful to the medical word as well as extremely lucrative; especially since the combined legal and illegal opiate market brought in revenues around $70 billion in 2009.[15]
References
| ||||||||||||