Mycobacterium tuberculosis ArfA Rv0899
From Proteopedia
IntroductionThe Rv0899 ArfA protein [ARFA_MYCTU] from Mycobacterium tuberculosis H37Rv belongs to the OmpA (outer membrane protein A) family of outer membrane proteins and has been proposed to act as an outer membrane porin with ion channel activity and contributes to the bacterium's adaptation to the acidic environment in phagosome [1] during infection [1]. Rv0899 protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro. Two Mycobacterium tuberculosis H37Rv genes (Rv0900 [ARFB_MYCTU] and Rv0901 [ARFC_MYCTU] ) adjacent to Rv0899 also encode putative membrane proteins, and are found exclusively in association with Rv0899 in the same pathogenic mycobacteria, suggesting that the three may constitute an operon dedicated to a common function. Asparagine is the primary ammonia source for Mycobacterium tuberculosis H37Rv at acidic pH [2]. The deletion of this gene impairs the uptake of some water-soluble substances, such as serine, glucose and glycerol [3]. Structural highlights
Structure SectionThe 326-residue Rv0899 ArfA contains three domains: an N-terminal domain (M domain) (residues 1-72) which includes a sequence of 20 hydrophobic amino acids required for membrane translocation. Residues form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker. The central B domain folds with three parallel/antiparallel Alpha Helices packed against six parallel/antiparallel Beta Strands that form a flat beta-sheet. is Hydrophobic, while the exterior is Polar and predominantly acidic. The two subdomains are symmetric about α2, and their backbone atoms can be aligned with a RMSD [2] of 1.8Å, by performing a 180° rotation of either one around an axis normal to the 6-stranded β-sheet.
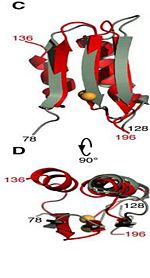
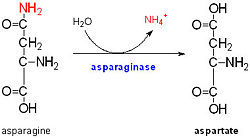
The C domain The C domain of wild-type ArfA into four Beta Strands and four Alpha Helices.Three parallel (β1, β2, β3) and one antiparallel (β4) β-strands form a four-stranded β-sheet (β1–β4-β2–β3) that packs against three α-helices (α1, α2, α3), while a fourth helix (α4) extends from the N-terminus of β4. The structure by disulfide bond between C208 and C250, by a network of hydrophobic contacts between α1, α2 and β4 (L211, I215, V243, L247, Ile323 and V325) between side chains and by a hydrogen bond between the backbone amide of V325 and the side-chain carbonyl of Q212. FunctionStress response of the bacterium and adaptation to the acidic external environment. 1. Acquisition of Zn(2+) ions by Ligand for DNA replication and transcription [4]. 2. Rapid ammonia secretion to external environment: deamidation of the amino acid pair , located at the end of α1 and preceding L3, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function [5]. 3. Stabilization of the outer membrane: 1. pH-dependent conformational dynamics of hydrophobic cluster of L232, F225, L240, A244, V281, L285 that folds to a more ordered structure like a flap at .
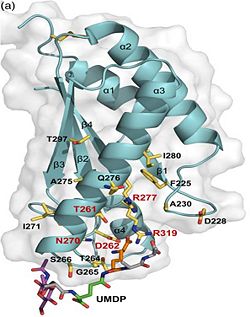
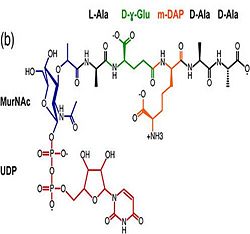
2. Binding to peptidoglycan and phospholipid layer. a) The B domain has homology with conserved putative (bacterial OsmY and nodulation) superfamily domains and conserved Gly95 and Gly164 [3], which putative functions are prevention of shrinkage of inner and outer membrane by binding to the phospholipid layer, nitrogen fixation and / or nitrogen metabolism [6]. b)The C domain has homology to the OmpA-C-like superfamily of periplasmic peptidoglycan-binding sequences, found in several types of bacterial membrane proteins.Contribution for structural strength to the bacterial cell wall under acid or other stress conditionsIts functions by binding to peptidoglycan biosyntheesis intermediate uridine-5-'-diphosphate-MurNAc–L-Ala–D-γ-Glu–m-DAP–D-Ala–D-Ala (UMDP) [4] binding site. . These residues are strictly conserved in the OmpA -like family. The side chain of m-DAP is stabilized by charge-charge interactions between its electronegative carbonyl group with R277 and R319 guanidinium groups and with the N270 carboxamide and between its electropositive amino group with the D262 carboxyl and the T261 hydroxyl. UMDP could interact through contacts of its γ-Glu3 and Ala2 backbone amides with the side-chain hydroxyl of S266, of its MurNAc O3 with the amide proton of E267, and of its MurNAc NH2 with the E267 carboxyl [7].
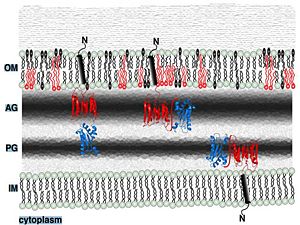
Putative localization in the outer membraneReferences
| ||||||||||||||||