Function
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a very important enzyme in the production of energy and in photosynthesis. In the production of energy this enzyme catalyzes the sixth step in the process of breaking down glucose, also known as glycolysis which occurs in organisms of all phyla. The sixth step consists of of the oxidation of GAP by NAD and an inorganic phosphate to yield 1,3 bisphosphoglycerate. In photosynthesis, which is carried out by plants and algae, this enzyme uses NADPH in the reverse reaction in a step in the Calvin cycle, which fixes gaseous CO2 into carbohydrate. Though these are its main functions, GAPDH has been shown to perform other functions including transcription activation, initiation of apoptosis, and ER to Golgi apparatus vesicle transportation [1]. However, this page will focus on GAPDH’s role in glycolysis. See 2pkq for the plant Calvin Cycle enzyme. See Glycolysis Enzymes and Carbon Fixation.
Structure
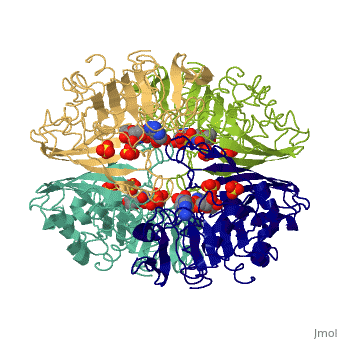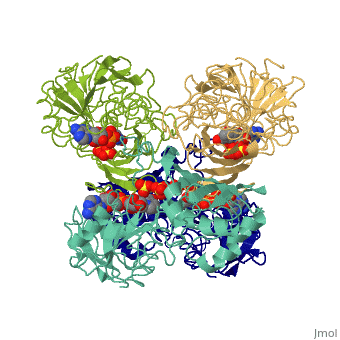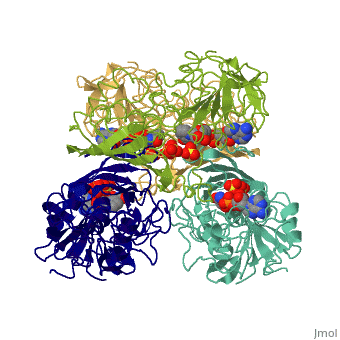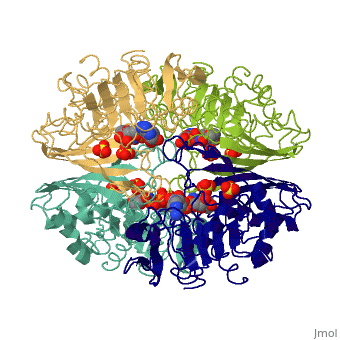
GAPDH most commonly exists as what looks to be a dimer. Interesting though, the two monomers of the enzyme are not exactly the same. While one side consists only of parallel and antiparallel beta-sheets, the other monomer is made up of both . Though each monomer does not have to exact same sequence, each does contain replicate active sites and function. This is consistent with the following SCOP information:
Class: Alpha and beta proteins (a/b)
Fold: NAD(P)-binding Rossmann-fold domains
Superfamily: NAD(P)-binding Rossmann-fold domains
Family: Glyceraldehyde-3-phosphate dehydrogenase-like, N-terminus domain
Protein: Glyceraldehyde-3-phosphate dehydrogenase
Species: Human
The specific reaction that GAPDH catalyzes is shown below:
GAP + NAD+ + Pi +GAPDH <==> 1,3-bisphosphoglycerate + NADH + H
Regulation
GAPDH is not a highly regulated step with in the glycolytic pathway because of relatively low energy transitions that occur between steps five and nine. However, there are a few items that must be present for the reaction to proceed. GAPDH is inactivated by the alkylation of iodoacetate, in which the Iodine inhibits the active site by binding to the Sulfur present in the cystine residue. If the active site is blocked by by an inhibitor such as Iodine then the reaction will stop. Also NAD oxidizes the GAP, so the availability of hydrogen and inorganic phosphates also could effect the rates of the reaction. The reason this step is not highly regulated is because Iodine is not present in the blood stream and the absence of both hydrogen and inorganic phosphates will cause the reaction to yield before this step is met.
Reaction Mechanism
The mechanism of the glycolysis reaction is fairly straight forward. After the aldehyde enters the (highlighted in green), the sulfhydryl group from attacks the nucleophilic carbon to form a thiohemiacetal. This intermediate undergoes oxidation due to a hydride transfer to a nearby NAD+ forming a thioester. From here, a phosphate group enters and attacks the same carbonyl while at the same time it is separated from the cystine by the protonated group. This produces the desired 1,3-bisphosphoglycerate. Though cysteine-151 and histidine-178 are direct contributers to the catalytic process, other residues also influence the activity of this enzyme indirectly. are two such residues that contribute to the binding of the reactants rather than the catalytic mechanism. Regulation of GAPDH occurs through its coupling with the PGK reaction. This coupling is needed due to the slightly positive delta G of the glycolysis. The larger negative delta G of the PGK reaction results in the following overall net reaction with a delta G of -12.1 kJ/mol:
GAP + Pi + NAD+ + ADP ==> 3PG + NADH + ATP
Additional Resources
For additional information, see: Carbohydrate Metabolism
3D structures of glyceraldehyde-3-phosphate dehydrogenase
Glyceraldehyde-3-phosphate dehydrogenase 3D structures