Methotrexate, formerly known as amethopterin, is a drug that is used in the competitive inhibition of Dihydrofolate reductase, resulting in decreased synthesis of dTTP and diminished cellular replication. The antimetabolic nature of methotrexate is most effective against the most rapidly dividing cells, making this drug useful in Cancer treatment, and various autoimmune diseases[1].
Chemical Properties
Chemical Formula: C20H22N8O5
Molecular Weight: 454.44 g/mol
Half-life: 3–15 hours[2]
History
Methotrexate has provided as a treatment option in clinical setting since the year 1948. Leukemia patients whom received folic acid, were observed to decline, while patients with restricted folic acid consumption improved, prompting experiments with analogs of folic acids. Methotrexate was originally developed from these observations suggesting that an analog of folic acid was able to cause a remission in symptoms of acute lymphoblastic leukemia in 1947. The subsequent derivation of a mechanism of action for methotrexate was developed and methotrexate was used for treatment of various cancerous even non-cancerous cases[3].
Structural Features DHFR
Human DHFR can be visualized as its . DHFR contains 4 alpha helical regions and 8 beta sheets as can be seen in its . The can also be seen. Human DHFR catalyzes the reduction of dihydrofolic acid to tetrahydrofolic acid, with NADPH serving as the electron donor in this reaction.
The can be seen with the residues that facilitate substrate binding and reaction process. NADPH and folate can both be seen interacting with the DHFR enzyme [4].
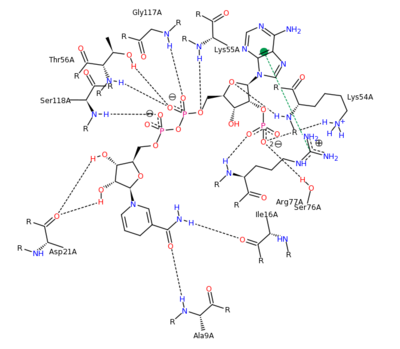
NADPH Residue Interaction
[5]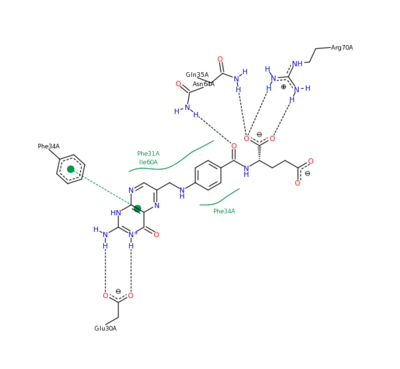
Folate Residue Interaction
[6]
Thymidine Synthesis Mechanism
[7] Biological Role of Dihydrofolate Reductase and Antifolates
In nucleotide metabolism, Thymine is formed through the methylation of dUMP resulting in dTMP (thymidylate), which can undergo phosphorylation forming dTTP, deoxyribose thymine triphosphate, commonly utilized in DNA synthesis and replication. Thymidylate synthesis is performed by thymidylate synthase, obtaining the methyl for the reaction from N5N10-methylene-tetrahydrofolate. Thymidylate synthase involves the oxidation of N5N10-methylene-THF forming the dihydrofolate (DHF) product through the transfer and reduction of the methylene to the methyl group of thymidylate, dTMP. While this dTMP generation reaction is crucial in nucleotide metabolism, equally important is the regeneration of the THF cofactor from the DHF product.
Dihydrofolate reductase (DHFR) is responsible for the reduction reaction that regenerates THF from DHF using NADPH. The subsequent act of serine hydroxymethyltransferase yields the starting N5N10- methylene-THF. DHFR is a biologically important molecule in the synthesis of dTMP and cell replication, and the inhibition of this enzyme halts dTMP synthesis. DHFR in most species occurs as an enzyme monomeric and monofuctional in nature; however, DHFR and thymidylate synthase are present in the form of a bifunctional enzyme in rare cases.
The relevance of dTMP synthesis in cellular replication overall makes it an important enzyme in cellular development and proliferation. The most rapidly replicating cells are most quickly utilizing their dTMP supply and therefore rely on the enzymes involved in dTMP synthesis more than slower growing cells. This increased dependence on these enzymes is accompanied by an increase sensitivity to their inhibition. This concept makes the enzymes involved in thmidylate synthesis such as dihydrofolate reductase, prime targets for cancer therapy. Inhibition of DHFR would result in the most rapidly replicating cells, cancer in most cases, rendered incapable of reproducing and eventual susceptible to cellular death. Antifolates are classified as molecules involved in blocking folic acid activity, and are in fact used in the cancer treatment[8].
Mechanism of Action
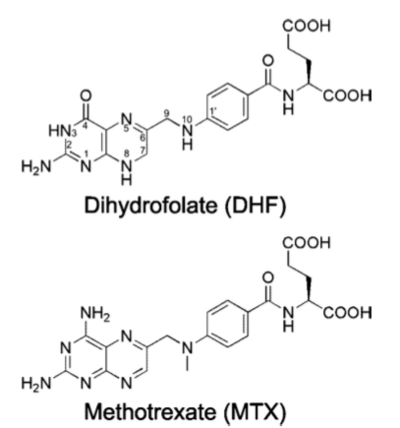
Methotrexate, is an antifolate which plays an inhibiting role in the synthesis of thymidylate through the prevention of THF regeneration. Methotrexate is a slow and tight binding competitive inhibitor of , resulting in the prevention of important metabolites necessary in thymidylate synthesis and nucleotide metabolism. Specifically, methotrexate acts as a DHF analog and through competitive inhibition of the DHFR active site, prevents the regeneration reaction necessary for further nucleotide biosynthesis. Methotrexate’s antimetabolite function seen in the competitive inhibition mechanism affects the metabolism of folic acid. Methotrexate is phase specific to the S phase of the cell cycle inhibiting DNA synthesis and replication within the afflicted cell. Competitive inhibition of the DHFR active site is possible because of the close resemblance that methotrexate shares with the metabolite being interfered with, dihydrofolate[9].
Folic Acid (left) Methotrexate (right)
The nature of this binding has a 1000 fold increase in affinity relative to the natural folate affinity of DHFR, producing a practically irreversible inhibition of DHFR activity. Methotrexate is a competitive inhibitor that can bind to and inhibit the (1u72). The . The are color depictions of each atom in regards to mobility or position uncertainty relative to the molecule, with increasing mobility as the color scheme goes from blue to red.
Experimental Mutation
The features of DHFR ligand binding, specifically to methotrexate can be observed and analyzed through various molecular docking and mutation experiments. The a structurally engineered variant of the (1u72) altered the (3eig) in an attempt to explore the specifics of the methotrexate affinity for DHFR active site residues, resulting in varied active site residues from phenylalanine and glutamine to arginine and glutamate. This mutated enzyme featured a 650x decrease in affinity for the ligand, methotrexate, but retained an amount of methotrexate interaction similar to the enzyme in its native state with native substrates. Crystal structure analysis revealed that the lack of cooperative action and presence of residue disorder lead to the significant decrease in methotrexate activity with the . The arginine residue at place 31, was specifically observed in numerous conformations, a characteristic unique to the mutated enzyme, and the probable cause of the loss of polar contacts and binding affinity between methotrexate and DHFR. A loss of van der Waal forces due to the conformations of the side chains along with an unfavorable placement of Glu-35 causing an “unfavorable electrostatic contact” with methotrexate’s “glutamate portion.” Interestingly this variant was found to display a greater decrease in methotrexate affinity than the decrease in affinity of Dihydrofolate, found to be 9x, evident of catalytic efficiency retention which hold many drug binding resistance implications[12].
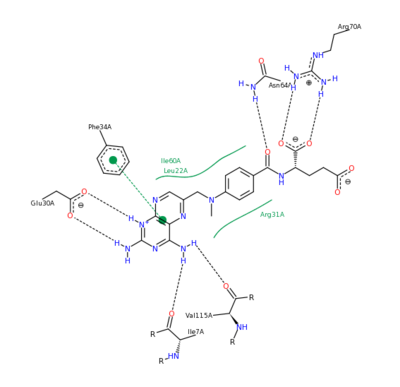
Methotrexate Variant Residue Interaction
[13] Pharmaceutical Implications
Methotrexate’s inhibition of cellular replication causes it to have an increased toxic response on cells performing DNA replication especially rapidly proliferating cells. These cells display decreased growth and division due to a lack of nucleoside biosynthesis metabolites, resulting in decreased dTMP. Methotrexate is able to interfere with rapid cell growth in this manner, specifically infecting cells including skin cells, bone marrow cells, and often cancer cells, making methotrexate an effective cancer treatment drug. Other DHF analogs exist which can be useful as anticancer agents or antibacterial agents, through inhibition of DHFR
[14].
Drug Treatment
Methotrexate is often used in a regimental approach to the chemotherapeutic treatment of cancers and other diseases involving replicating tissue. A variety of specific cancer types have been treated with methotrexate including head, lung, skin, or breast cancer. Methotrexate has also been used in the treatment of various autoimmune diseases. Rheumatoid arthritis and psoriasis have also utilized methotrexate as a treatment method, presumably to diminish immune function. The mechanism of methotrexate in these instances varies from the inhibition of DHFR, but involves inhibition of enzymes involved with purine metabolism resulting in various types of immune suppression including inhibition of T cell activation. Because of this altered mechanism, treated patients are often administered folate to offset the antifolate characteristics of methotrexate. The targeting of rapidly replicating cells allows methotrexate to function as an abortifacient as well. These uses of methotrexate need to be carefully monitored with proper dosage because methotrexate is embryotoxic, carcinogenic, and teratogenic[15].
Trexall is a drug, methotrexate tablet, used as an antimetabolite for treatment of neoplastic diseases, severe rheumatoid arthritis and psoriasis[16].
Treatment
Methotrexate can be administered orally as well as intravenous, intramuscular, subcutaneous, or intrathecal injection. Dosage amount is a crucial aspect of any methyltrexate treatment because of the serious side affects, and often results in dosages being taken rarely more than once or twice a week. The immune system, blood cells, and other rapidly replicating cells including liver, lungs, kidneys are often succeptable to damage which requires regular tests. Side effects from this drug can be common and sever including neutropenia, hair loss, nausea, dermatitis, and anemia, often representative of the antimetabolite function of methotrexate. Stomatitis is not commonly seen with weekly doses, but daily doses for 5 consecutive days often results in these symptoms including renal impairment, toxicity, and possible failure. Myelosuppression may develop with increased dosages, enhancing tissue damage resulting most commonly from radiation of cancer patients. Additional drugs including antibiotics can often result in adverse side effects, and increased methotrexate retention due to additional drugs can often lead to a dangerous increase in concentration of methotrexate in the blood
[17].
The nature of this treatment type can requires the “rescue” of a patient, through withdrawal of the inhibitor and possible administering of thymidine or folic acid based drugs to prevent the toxicity sometimes seen in beneficial rapidly replicating cells. Leucovorin is often administered for this rescuing effect. Leucovorin or folinic acid is a derivative of THF, and can be readily converted to tetrahydrofolate overcoming the effect of methotrexate because it bypasses the dihydrofolate reductase mechanism to produce THF[18].
Pharmacokinetics
Dosage size of methotrexate is extremely important because of the antimetabolic function of the drug, therefore many pharmacokinetic properties must be considered prior to treatment. Methotrexate is a dicarboxylic acid, although with a pKa of 4.8 and 5.5 is weak and often ionized in physiological conditions. Bioavailability following oral absorption is dose dependent, with 60 percent at doses lower than 30 mg/m2, and at concentrations above 80 mg/m2, there is only 20 percent bioavailability, percentages that can be increased with intramuscular administering of the drug. Only about 5 percent of the total loss of the oral dose is due to bacterial degradation. The kidney, spleen, liver, gallbladder, as well as the skin display the highest levels of methotrexate upon treatment. This drug does not cross the blood brain barrier efficiently, but the distribution to the kidney and liver may be prolonged with higher doses extending drug clearance time. Methotrexate can be metabolized through the liver and intracellular mechanisms, and the kidneys are capable of excreting from 80 to 90 percent of the drug without metabolizing methotrexate[19].
Additional Information
For additional information see: Pharmaceutical Drugs