We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
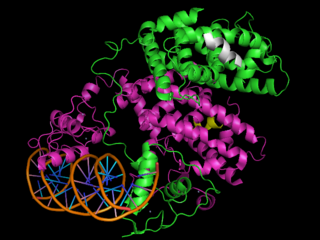
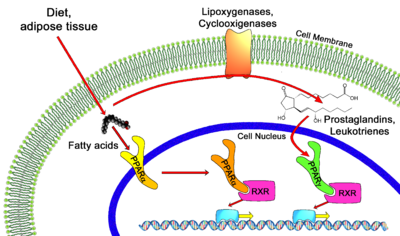
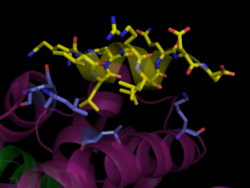
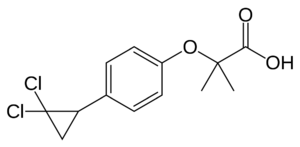
Peroxisome Proliferator-Activated Receptors
From Proteopedia
(Redirected from Peroxisome Proliferator-Activated Receptor)
| |||||||||||
Additional Resources
- See: Regulation of Gene Expression For Additional Mechanisms of Gene Regulation
- See: Pharmaceutical Drug Targets For Additional Information about Drug Targets for Related Diseases
- See: Diabetes & Hypoglycemia For Additional Information about Diabetes & Hypoglycemia Related Information
References
- ↑ 1.0 1.1 1.2 1.3 Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-35. PMID:11818483 doi:10.1146/annurev.med.53.082901.104018
- ↑ van Raalte DH, Li M, Pritchard PH, Wasan KM. Peroxisome proliferator-activated receptor (PPAR)-alpha: a pharmacological target with a promising future. Pharm Res. 2004 Sep;21(9):1531-8. PMID:15497675
- ↑ Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289-312. doi: 10.1146/annurev.biochem.77.061307.091829. PMID:18518822 doi:http://dx.doi.org/10.1146/annurev.biochem.77.061307.091829
- ↑ Lagathu C, Kim M, Maachi M, Vigouroux C, Cervera P, Capeau J, Caron M, Bastard JP. HIV antiretroviral treatment alters adipokine expression and insulin sensitivity of adipose tissue in vitro and in vivo. Biochimie. 2005 Jan;87(1):65-71. PMID:15733739 doi:http://dx.doi.org/10.1016/j.biochi.2004.12.007
- ↑ Qi C, Zhu Y, Reddy JK. Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochem Biophys. 2000;32 Spring:187-204. PMID:11330046
- ↑ Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev. 2005 Feb 15;19(4):453-61. Epub 2005 Jan 28. PMID:15681609 doi:10.1101/gad.1263305
- ↑ Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995 Jun;15(6):3012-22. PMID:7539101
- ↑ Lee SK, Jung SY, Kim YS, Na SY, Lee YC, Lee JW. Two distinct nuclear receptor-interaction domains and CREB-binding protein-dependent transactivation function of activating signal cointegrator-2. Mol Endocrinol. 2001 Feb;15(2):241-54. PMID:11158331
- ↑ Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999 Jun 25;284(5423):2174-7. PMID:10381882
- ↑ Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997 Jul 25;272(30):18779-89. PMID:9228052
- ↑ Girroir EE, Hollingshead HE, He P, Zhu B, Perdew GH, Peters JM. Quantitative expression patterns of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) protein in mice. Biochem Biophys Res Commun. 2008 Jul 4;371(3):456-61. Epub 2008 Apr 28. PMID:18442472 doi:10.1016/j.bbrc.2008.04.086
- ↑ Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7473-8. PMID:10377439
- ↑ Gottlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4653-7. PMID:1316614
- ↑ Amri EZ, Bonino F, Ailhaud G, Abumrad NA, Grimaldi PA. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem. 1995 Feb 3;270(5):2367-71. PMID:7836471
- ↑ 15.0 15.1 15.2 Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998 Sep 10;395(6698):137-43. PMID:9744270 doi:10.1038/25931
- ↑ Fyffe SA, Alphey MS, Buetow L, Smith TK, Ferguson MA, Sorensen MD, Bjorkling F, Hunter WN. Recombinant human PPAR-beta/delta ligand-binding domain is locked in an activated conformation by endogenous fatty acids. J Mol Biol. 2006 Mar 3;356(4):1005-13. Epub 2006 Jan 4. PMID:16405912 doi:10.1016/j.jmb.2005.12.047
- ↑ Yang W, Rachez C, Freedman LP. Discrete roles for peroxisome proliferator-activated receptor gamma and retinoid X receptor in recruiting nuclear receptor coactivators. Mol Cell Biol. 2000 Nov;20(21):8008-17. PMID:11027271
- ↑ 18.0 18.1 18.2 18.3 18.4 Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta. 2007 Aug;1771(8):915-25. Epub 2007 Jan 18. PMID:17317294 doi:10.1016/j.bbalip.2007.01.007
- ↑ 19.0 19.1 19.2 Gampe RT Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000 Mar;5(3):545-55. PMID:10882139
- ↑ Xu HE, Lambert MH, Montana VG, Plunket KD, Moore LB, Collins JL, Oplinger JA, Kliewer SA, Gampe RT Jr, McKee DD, Moore JT, Willson TM. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13919-24. Epub 2001 Nov 6. PMID:11698662 doi:10.1073/pnas.241410198
- ↑ Wahli W, Braissant O, Desvergne B. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more.... Chem Biol. 1995 May;2(5):261-6. PMID:9383428
- ↑ Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008 Nov 20;456(7220):350-6. PMID:19043829 doi:10.1038/nature07413
- ↑ 23.0 23.1 Berger J, Wagner JA. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol Ther. 2002;4(2):163-74. PMID:12079620
- ↑ http://uk.reuters.com/article/idUKT7482820080131
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Alexander Berchansky, Joel L. Sussman