SCF-KIT
From Proteopedia
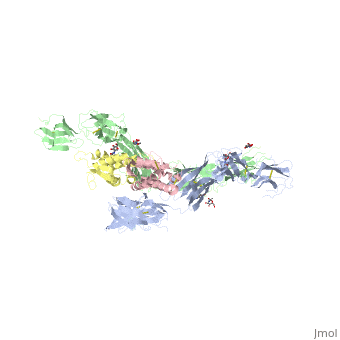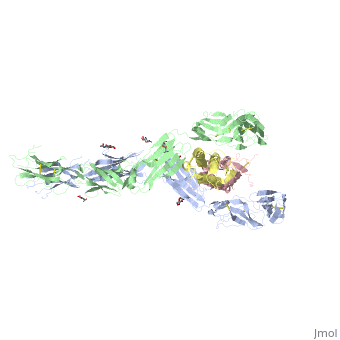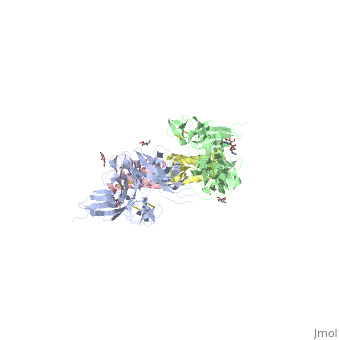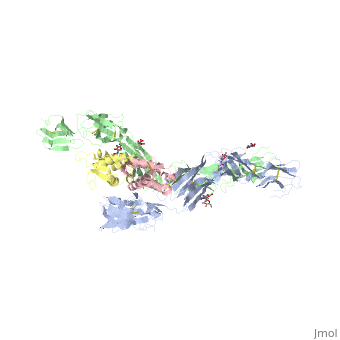
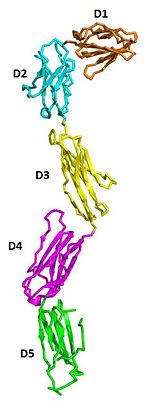
IntroductionStem cell factor (SCF) is a cytokine that mediates its diverse cellular responses by binding to and activating the receptor tyrosine kinase (RTK). KIT (also known as SCFreceptor), functions as a noncovalent homodimer, and both membrane-anchored and soluble forms of SCF have been described. KIT was initially discovered as an oncogene in a feline retrovirus. SCF and KIT are required for a normal development of hematopoietic cells, melanocytes and others. In humans, loss-of-function mutations in KIT cause the piebald trait. A variety of gain-of-function mutations in KIT were found in different types of human cancers such as gastro-intestinal-stromal tumors, acute myeloid leukemia, and mast cell leukemia. StructureThe two sets of KIT ectodomains and SCF molecules resemble an upside down ‘‘A’’ letter and the entire ectodomain of KIT is composed of five Ig-like domains: D1-D5. SCF dimer interacts symmetrically with D1, D2, and D3 of two corresponding KIT ectodomains. In addition, KIT ectodomains form homophylic interactions through lateral contacts between D4 and D5 of the two neighboring receptors. The folding of the 5 KIT domains is a typical folding to the immunoglobulin super family. Structural changes upon SCF binding to KIT: The D1-D2-D3 region of KIT is a functional unit that is poised for SCF binding, thus leading to subsequent KIT dimerization which is driven by dimeric SCF molecules. In addition, while the overall structure of SCF bound to KIT is similar to the structure of , there are differences in the angle between the two protomers, in the conformations of the connecting loops and in the structures of the flexible N terminus of the molecule. The structure is also characterized by the existence of a large cavity at the center of the complex. Each protomer of SCF binds to a single KIT molecule and that receptor dimerization is driven by SCF dimers, leading to additional receptor-receptor interactions.
FunctionDimerization of KIT is also mediated by homotypic interactions between the two membrane-proximal Ig-like domains of KIT, namely by D4-D4 and D5-D5 interactions. This results in a significant change in the configurations of D4 and D5 relative to the rest of the molecule. These configurations bring the C termini of the two neighboring ectodomains within 15 Å of each other, close to the place where they connect to the transmembrane domain. SCF-KIT interface can be divided to three: SiteI, Site II and Site III. The dimerization of KIT is made possible by whose sole function is to bind SCF and to bring together two KIT molecules. This dimerization is followed by a large change in D4 and D5 orientations. It was proposed that the flexible joints at the D3-D4 and D4-D5 interfaces enable lateral interactions that result in a large conformational change upon receptor dimerization. In the process of dimerization, there is a selection between particular conformations in a transition from a flexibly jointed monomer to a rigid dimer. A similar mechanism was found in VEGFR, a protein that takes part in regulation of blood and lymphatic vessel development and homeostasis.
MutationsA point mutation in either within D4 can strongly compromise SCF-induced tyrosine autophosphorylation. The homotypic interactions between membrane-proximal regions of KIT are mediated primarily by the D4-D4 interface, while the D5-D5 interface plays a cooperative secondary role, i.e., the D5-D5 faciliates the exact positioning of two KIT ectodomains at the cell-surface interface. SCF-KIT binding occurs in at least two steps: First, the electrostatic attraction between SCF and D1-D2-D3 will align SCF along the opposing ligand-binding region on KIT. A faster association rate occurs as a result of an electrostatic attraction of SCF due to a steering effect. Interestingly, the main interactions that maintain the D4-D4 interface, i.e. double salt bridges between Arg381 and Glu386 in a neighboring KIT molecule are also mediated by electrostatic interactions. Since the hallmarks of KIT structure, ligand binding and receptor dimerization are conserved in other receptors, it is believed that the mechanism described above for KIT activation is a general mechanism for activation of many receptors. 3D structure of SCFSee Tyrosine kinase References
| ||||||||||||