Semaglutide
From Proteopedia
Semaglutide is an analog of the GLP-1 (glucagon-like peptide 1) hormone. It acts as an agonist to the GLP-1 receptor (GLP-1R) and is used as drug to manage diabetes. Its use has been growing as a weight-loss medication, and potential benefits across a wide range of diseases is currently studied.
Contents |
Discovery
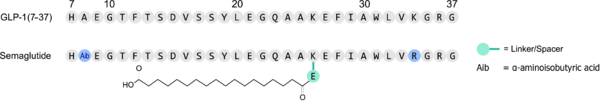
Semaglutide was discovered [1] in an effort to increase the lifetime of a once-a-day medication called Liraglutide. It is derived from the GLP-1 hormone with modifications described below.
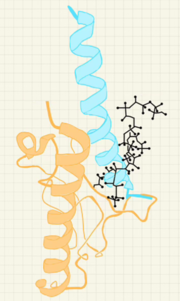
Structure and binding to GLP-1R
| |||||||||||
Synthesis
Semaglutide is made by Novo Nordisk, and the details are proprietary and protected by patents[4]. The product is semisynthetic, with the bulk of the peptide made in recombinant yeast[5] and the fatty acid and the first two amino acids attached afterwards. Because the second lysine of GLP-1 is replaced by an arginine in semaglutide, the fatty acid is attached to a single lysine, simplifying the coupling between peptide and linker-attached fatty acid.
Medical use of Semaglutide
Semaglutide medications (Ozempic, Wegovy, and Rybelsus) have become extremely popular over time in managing weight loss and obesity. A KFF poll conducted in May 2024 found that 1 in 8 respondents (adults in the United States) say that they have taken a GLP-1 agonist in the past [6]. Originally developed as treatments for type 2 diabetes, medications such as Ozempic are now widely used for their ability to aid weight loss. As a GLP-1 receptor agonist (GLP-1 RA), semaglutide works by mimicking the effects of the GLP-1 hormone in the body. This action helps regulate the metabolic system, control appetite, and reduce calorie intake, leading to significant weight loss in many users. Semaglutide also slows gastric emptying, which prolongs the feeling of fullness after eating and further supports weight management.
Side effects
While semaglutide has shown great promise in helping manage obesity, it is associated with a variety of dose-dependent side effects, as demonstrated in clinical trials. The most commonly reported adverse effects are gastrointestinal and neurological in nature. Gastrointestinal issues include nausea, diarrhea, vomiting, constipation, and abdominal pain. These effects are primarily due to semaglutide's mechanism of action, as the slowing of gastric emptying can cause discomfort, particularly at higher doses.[7]
Neurological side effects, including headaches and dizziness, are also frequently reported. These may result from fluctuations in blood sugar levels and how the brain adjusts to changes in hunger and energy signaling. Other less direct effects, such as gastroenteritis, may occur due to altered gut motility or changes in gut flora, which increase susceptibility to infection. Additionally, semaglutide can affect kidney function because reduced fluid intake may lead to dehydration, highlighting the importance of monitoring hydration levels. Some users also report nasopharyngitis, potentially linked to immune modulation, though this is less common.
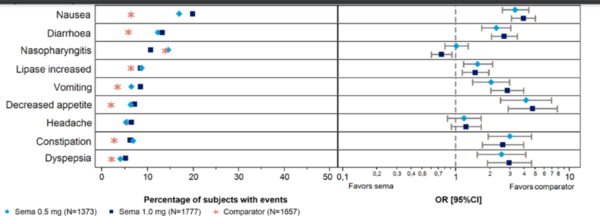
The bar chart depicts the proportion of patients experiencing common adverse events (AEs) during Phase 3a trials for semaglutide (0.5 mg and 1.0 mg) compared to other treatments, including exenatide ER and insulin glargine. The AEs include gastrointestinal disorders (nausea, vomiting, diarrhea, constipation), nasopharyngitis, fatigue, and headache. Data are presented as adjusted percentages, with odds ratios (OR) and confidence intervals (CI) providing additional context. OR values above 1.0 suggest a higher likelihood of AEs with semaglutide, while CIs indicate the range of variability and precision. The results show that semaglutide, particularly at the 1.0 mg dose, is associated with a higher frequency of gastrointestinal AEs compared to comparators. Neurological side effects, such as headache and fatigue, and nasopharyngitis were also more common in semaglutide groups. These findings underscore the dose-dependent nature of AEs with semaglutide and the importance of balancing its efficacy in weight loss with patient tolerability.[5]
While semaglutide is effective for weight loss, these findings emphasize the need to balance therapeutic benefits with patient tolerability, tailoring treatment plans to minimize side effects while achieving optimal outcomes.
Semaglutide, a GLP-1 receptor agonist, has demonstrated efficacy in weight loss and glycemic control but is often discontinued due to adverse effects and financial barriers. Real-world data reveal that 36.5% of patients discontinue GLP-1 agonists within 12 months, with higher rates among those with obesity compared to type 2 diabetes[8]. Factors such as gastrointestinal side effects, out-of-pocket costs, and demographic disparities, including race and socioeconomic status, significantly contribute to discontinuation rates (Do et al., 2024). These findings highlight the need for balancing therapeutic benefits with patient tolerability and accessibility.
Further development
Semaglutide targets a single receptor, GLP-1R. There are dual and triple agonists in development that target multiple receptors. Another avenue of development is to further increase the life-time of synthetic agonists, or to make conjugates that target a payload to cell that carry the GLP-1 receptor. One goal is to find agonists with fewer side effects, and another is to find alternative agonists that are better suited for oral uptake [9].
3D structures of semaglutide
Updated on 18-April-2025
7ki0 - hSG + GLP-1 receptor + Gs proteins + nobody - human - Cryo EM
7ki1 - hTaspoglutide + GLP-1 receptor + Gs proteins + nobody - human - Cryo EM
4zgm - hSG + GLP-1 receptor
Student contributors
This page was created as a two-week project of an undergraduate biochemistry course. Karsten Theis would like to acknowledge contributors to the Structure, Half-life and Side effects sections, Paige, Marissa, Faith McCormack, William Buckley, Humzah, Mahrosha, Evelyn, Naba Algertani, Faiza Abdullah, Sara, and Natalisha.
References
- ↑ 1.0 1.1 Lau J, Bloch P, Schaffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T. The discovery of the once weekly glucagon like peptide 1 (GLP-1) analog semaglutide. J Med Chem. 2015 Aug 26. PMID:26308095 doi:http://dx.doi.org/10.1021/acs.jmedchem.5b00726
- ↑ Zhang X, Belousoff MJ, Liang YL, Danev R, Sexton PM, Wootten D. Structure and dynamics of semaglutide complexes. Cell Rep. 2021 Jul 13;36(2):109374. PMID:34260945 doi:10.1016/j.celrep.2021.109374
- ↑ Röhrborn D, Wronkowitz N, Eckel J. DPP4 in Diabetes. Front Immunol. 2015 Jul 27;6:386. PMID:26284071 doi:10.3389/fimmu.2015.00386
- ↑ Alhiary R, Kesselheim AS, Gabriele S, Beall RF, Tu SS, Feldman WB. Patents and Regulatory Exclusivities on GLP-1 Receptor Agonists. JAMA. 2023 Aug 15;330(7):650-657. PMID:37505513 doi:10.1001/jama.2023.13872
- ↑ 5.0 5.1 Committee for Medicinal Products for Human use (CHMP). European Medicines Agency (EMA). (2018). https://www.ema.europa.eu/en/documents/assessment-report/ozempic-epar-public-assessment-report_en.pdf
- ↑ Harris E. Poll: Roughly 12% of US Adults Have Used a GLP-1 Drug, Even If Unaffordable. JAMA. 2024;332(1):8. DOI:10.1001/jama.2024.10333
- ↑ Fornes A, Huff J, Pritchard RI, Godfrey M. Once-Weekly Semaglutide for Weight Management: A Clinical Review. J Pharm Technol. 2022 Aug;38(4):239-246. PMID:35832567 doi:10.1177/87551225221092681
- ↑ Do D, Lee T, Peasah SK, Good CB, Inneh A, Patel U. GLP-1 Receptor Agonist Discontinuation Among Patients With Obesity and/or Type 2 Diabetes. JAMA Netw Open. 2024 May 1;7(5):e2413172. PMID:38787563 doi:10.1001/jamanetworkopen.2024.13172
- ↑ Novikoff, Aaron et al. Why are we still in need for novel anti-obesity medications? The Lancet Regional Health – Europe, Volume 47, 101098 DOI:10.1016/j.lanepe.2024.101098