Stereochemistry
From Proteopedia
Stereochemistry refers to the three dimensional arrangement of atoms in molecules, especially those aspects that go beyond the connectivity (which atoms are connected to each other) captured in a Lewis structure. Two molecules that are mirror images of each other (enantiomers) differ in stereochemistry. Another common example are cis and trans double bonds, such as in fatty acids.
Contents |
Chirality
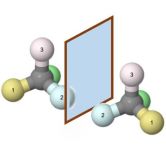
Chiral molecules are like your hands. There are two versions, left and right, and they are mirror images (or enantiomers). The initial scenes shows how four different substituents on a tetrahedral center leads to two possible configurations. It is impossible to superimpose the two molecules without breaking some of the bonds (i.e. swapping two of the substituents).
If you make a fist with your right hand and hold out your left hand flat, they are still considered mirror images because a hand is still a hand, even when you make a fist. Similarly, you sometimes have to rotate around some single bonds to have a pair of more complex enantiomers actually look like mirror images.
|
|
Explore it yourself
You can rotate the left and the right molecule by dragging them. Try rotating the right molecule so that is looks exactly the same as the left molecule. Or imagine a mirror between the two molecules, and rotate them that the same atoms are on top and on the bottom, the same atoms are in front and in the back, but left and right is reversed.
Other forms of stereo chemistry
Clicking on the buttons below loads other pairs of molecules. Each pair somehow is different in stereochemistry. For each pair, try to write a Lewis structure and a skeletal structure, and describe how they differ.