T4 RNA ligase 2 (Rnl2)
From Proteopedia
Overview:T4 RNA ligase 2 (Rnl2) (1-249) is a T4 bacteriophage ligase, which functions to counter bacterial defence by repairing broken anticodon loops of tRNA coding for lysine, caused by PrrC, which is necessary for viral replication and proliferation[1]. Note that the structure shown is based on the truncated enzyme form (1-249) where the total chain length of Rnl2 is 334 residues. The overall structure, at 100K, pH 8.5, consists of a single chain polymer (233 residues + 15 His tag residues) composed of 2, 6 antiparalel stranded, beta sheets and 7 alpha helices [1]. The structure contains an adenosine monophosphate ligand in its active site. The enzyme contains 5 nucleotidyl transferase motifs,I, III, IIIa, IV and V (green, blue, pink, light blue, red) which are all involved either directly or indirectly with the function of the active site [1].
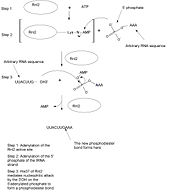
Protein Function:Although this protein is designed to counteract bacterial host defence mechanisms against the virus, its activity as a ligase is analogous to tRNA splicing (introns removed from anticodon loop) and RNA editing by several different kinds of RNA editing ligases (REL)s [1]. Therefore, it has been grouped into a subfamily of RNA ligases including REL-1 which is essential for the survival of trypanosoma [1], a monophyletic group of unicellular parasitic flagella protozoa that causes sleeping sickness. Another commonality shared between RNA ligases as well as DNA ligases and capping enzymes is the fact that the mechanism by which they function involves the formation of a covalent enzyme-(lysyl-N)-NMP intermediate prior to the formation of the phophodiester bond between 5’ and 3’ ends of RNA strands [1]. Hence these groups have been classified as a super family of enzymes, characteristic of the above intermediate. The mechanism for the formation of this intermediate and the phosphodiester bond resulting in RNA strand repair is outlined below (Figure 1). First ATP reacts with the active site of Rln2. Adenosine monophosphate (AMP) binds to the active site (light green) accompanied by the release of inorganic phosphate which provides the energy [1]. The adenylate binds at the bottom of the active site (motif 1) where it is squished between the aromatic rings of Phe 119 (light blue) of motif IIIa, Val 207 (purple) of motif III and Lys 35 (yellow) of motif I. Lys 225 (orange) and Lys 227 (peach) of motif V, which are essential for Rln2 activity, are also involved in coordinating the AMP phosphate after binding [1]. During ligase adenylation the alpha phosphate of ATP is thought to be stabilized by Lys 225 and 227 prior to release of the other phosphate groups [1]. The specificity of Rln2 for ATP as opposed to other NTP substrates in this step are thought to be attributed to hydrogen bonding between backbone residues and the adenine base [1]. Such bonds occur between:N7 (on AMP) and the backbone amide of Ile36 (dark green), the exocyclic 6-amino group (N6) and main chain carbonyl (O) of Glu34 (dark purple), N1 and Lys 209 (dark red) (water (white circles) mediated) [1]. After Binding AMP, the Rnl2 transfers AMP to the 5' phosphate of the RNA strand. The binding of Rnl2 to RNA, required for this step, can be partially attributed to the electrostatic structure of the enzyme [1]. Since the active site surface surrounding AMP is positively charged, it may contribute to the affinity of the enzyme for the negatively charged 5' phosphate of pRNA [1]. Note that all interactions between pRNA and Rnl2 also require the essential C-terminal domain which is not shown in the crystalized structure here [1]. This may be due to the essential positioning requirements for the placement of ATP in correct close proximity and orientation to the 5' phosphate for the next step (Figure 1) [1]. The interactions between the ribose sugar and the protein back bone are all thought to contribute to this adenyltransferase function as well as overall pRNA ligation, where the interaction between the 2' oxygen of the ribose sugar and Glu 99 (teal) is thought to be most essential to this step [1]. In addition, the Arg55 (dark blue) guanidinium group (NH2, NE, NH1), which coordinates the 3' O of the ribose sugar, is thought to interact with the 5'-PO4 of RNA during this adenylation step as well as during initial adenylation of the enzyme by ATP (Figure 2) [1]. Likewise Glu 204 has been found to be essential to both of these steps as well [1]. The π stacking between Phe119 and the adenylate base is thought to be critical for the next step; transfer of the phosphate from the 5’ end of the RNA strand to the 3’ OH end of the RNA strand to form the phosphodiester bond (Figure 2). The actual catalytic action of this step however, is thought to be carried out by His 37 (light green) in motif 1 [1]. Ho et al. hypothesized that His 37 sets the boundaries of the binding site for the 3' OH RNA strand which acts as a nucleophile in this step and hence is necessary for catalyzing this step [1]. It has also been found that residues Asp 120 and Lys 209 are also both essential for Rnl2 activity even though they are not in direct association with AMP itself [1].
Structural Components Contributing to the Active Site:Structural components contributing to the Rnl2 active site Above the components of the actual active site were discussed in relation to the function of the protein, however there are several indirect structural components which are linked to maintaining active site structure as well as aiding in conformational changes while it carries out its function. For instance Asp 120 (silver) of motif IIIa (pink), located next to Phe 119 (see above for function), serves as a focal point of hydrophilic contacts which include a bidentate salt bridge to Arg 221 (dark green) and a hydrogen bond to Tyr 5 (dark purple). These structural components tether 4 beta strands that make up the nucleotide binding pocket of Rnl2. click here to see H-bonding patterns between side chains [1]. Glu 34 (dark red) has also been found to be structurally important, although not making direct contact with AMP, as it functions to make sure that the loop containing the lysine nucleophile is in proper conformation for adenylation [1]. It accomplishes this structural function by tethering the backside of the motif I loop (green) to the start ofalpha helix 6 (lime green) through a hydrogen bond to Ser 170 (dark purple) [1].
Significance of T4 Rnl2 to the medical industry:Trypanosomaisis affects between 300, 000 to 500, 000 people in rural parts of Central Africa every year in which almost all cases are fatal and currently no effective treatment method exists for the disease.[2] Because of the several similarities Rnl2 shares with REL-1 and other RELs, in particular its active site and binding sites, an understanding of this protein's structure in relation to its function can give further insight as to how the REL's structure may relate to their function. If the enzymes behave similar mechanistically, target pharmaceuticals designed to inhibit the Rnl2 active site or binding sites may also serve as potential inhibitors against RELs. Since the RELs have been found to be essential to trypanosomiasis and leishmaniasis, mentioned earlier, they have become prime targets for drugs against these microorganisms [1]. Such drugs can be designed to mimic the substrate, bind to impair the AMP binding pocket, or bind to other surface residues which are essential to the function of the protein such as Glu 34, or His 37 [1]. |
| |||||||||||
3D structures of RNA ligase
References:
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 Ho CK, Wang LK, Lima CD, Shuman S. Structure and mechanism of RNA ligase. Structure. 2004 Feb;12(2):327-39. PMID:14962393 doi:10.1016/j.str.2004.01.011
- ↑ Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, Bohme U, Hannick L, Aslett MA, Shallom J, Marcello L, Hou L, Wickstead B, Alsmark UC, Arrowsmith C, Atkin RJ, Barron AJ, Bringaud F, Brooks K, Carrington M, Cherevach I, Chillingworth TJ, Churcher C, Clark LN, Corton CH, Cronin A, Davies RM, Doggett J, Djikeng A, Feldblyum T, Field MC, Fraser A, Goodhead I, Hance Z, Harper D, Harris BR, Hauser H, Hostetler J, Ivens A, Jagels K, Johnson D, Johnson J, Jones K, Kerhornou AX, Koo H, Larke N, Landfear S, Larkin C, Leech V, Line A, Lord A, Macleod A, Mooney PJ, Moule S, Martin DM, Morgan GW, Mungall K, Norbertczak H, Ormond D, Pai G, Peacock CS, Peterson J, Quail MA, Rabbinowitsch E, Rajandream MA, Reitter C, Salzberg SL, Sanders M, Schobel S, Sharp S, Simmonds M, Simpson AJ, Tallon L, Turner CM, Tait A, Tivey AR, Van Aken S, Walker D, Wanless D, Wang S, White B, White O, Whitehead S, Woodward J, Wortman J, Adams MD, Embley TM, Gull K, Ullu E, Barry JD, Fairlamb AH, Opperdoes F, Barrell BG, Donelson JE, Hall N, Fraser CM, Melville SE, El-Sayed NM. The genome of the African trypanosome Trypanosoma brucei. Science. 2005 Jul 15;309(5733):416-22. PMID:16020726 doi:309/5733/416
Proteopedia Page Contributors and Editors (what is this?)
William Eisbrenner, Alexander Berchansky, David Canner, Sonja Senekovic, Michal Harel, Andrea Gorrell