UBC13 MMS2
From Proteopedia
SummaryUbc13 is an E2 ubiquitin-conjugating enzyme that can form a heterodimer with Mms2 to function as a part of the translesion synthesis (TLS) pathway,[1][2]. When bound to Mms2, Ubc13 will polyubiquitinate proliferating cell nuclear antigen (PCNA), a sliding clamp protein at the DNA transcription fork[1]. Ubc13-Mms2 functions to polyubiquitinate PCNA following the initial monoubiquitination by Rad6-Rad18 (another E2 complex)[2]. It is important to note that Ubc13 lacks the ability to be catalytically active without Mms2, hinting at inaccuracies within the statement "structure determines function." FunctionUbc13 functions as a heterodimer with Mms2, a structurally similar protein to Ubc13 that lacks the catalytic cysteine residue in the active site[3][4]. The Ubc13-E2 complex with Mms2 functions primarily to enhance DNA repair from double stranded breaks. Mms2 bound to Ubc13 helps orient the ubiquitin molecule for proper ubiquitination of the K63 residue[5]. Mms2 is considered a Ubiquitin E2 variant (UEV) protein, because it lacks the catalytic cysteine residue necessary for proper thioester formation[5][6][3]. RegulationThe regulation of Ubc13 is controlled by the competitive binding of the two different UEV's, Mms2 and UEV1A[2]. Ubc13 binding to Mms2 activates the DNA repair pathway, while Ubc13 binding to UEV1A activates the NF-kappaB pathway, a gene regulation pathway involved in DNA transcription factors[2]. These two opposing pathways being downstream targets for Ubc13-bound complexes is the basis for regulation. Coordinating Enzymes
Pathway for DNA Repair
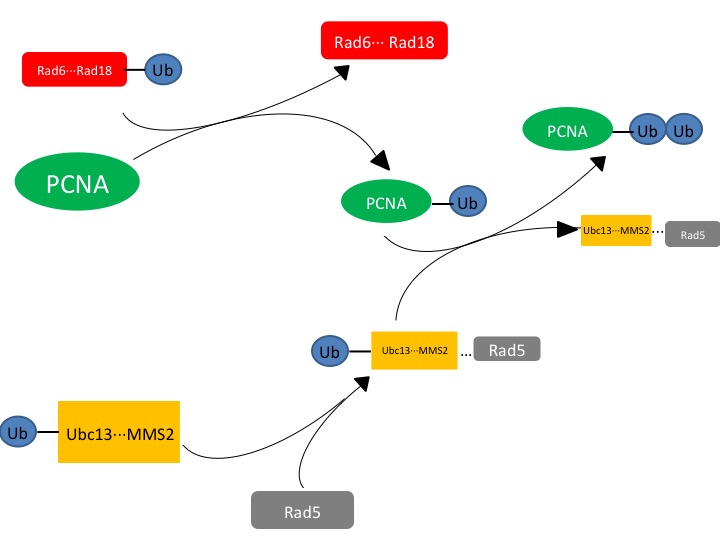
Figure. 1. The affected protein PCNA is a DNA clamp that is monoubiquitinated by the Rad6-Rad18 complex. The monoubiquitinated PCNA is then polyubuiquitinated by the UBC13-Mms2-Rad5 complex.
Structural Highlights/Important ResiduesUbc13 weights 17.6 kDA, and Mms2 is 16.8 kDA[4]. The heterodimer is stable at high stalt concentrations (1 M), suggesting strong interactions between the two. Kd between the Ubc13 and Mms2 is 2 uM. and of Ubc13 interact with the N-terminal domain of Mms2 to ensure stable docking[4]. Additionally, hydrophobically interacts with an alpha helix of Mms2 in two places[4]. Mms2’s is inserted between Glu55, Phe57, and Arg70 of Ubc13 to create a [4]. It is therorized that of Ubc13 are more important for recognition instead of stability[4]. MechanismThe exact mechanism for how Ubc13 transfers ubiquitin is not known, however the mechanism occurs in either a step-wise or concerted reaction. Ubc13, as an E2, froms a covalent bond with ubiquitin and then transfers the ubiquitin to the target protein via a thioester intermediate. Ubiquitin is removed from Ubc13 and Mms2 complex and placed onto PCNA. 3D structure of Ubc13References
| ||||||||||||
Proteopedia Page Contributors and Editors (what is this?)
David A Taves, Michal Harel, Christopher Alexander Hudson, Nicholas R. Dunham