User:Adam Kral/Sandbox 1
From Proteopedia
Contents |
Bruton's X-linked agammaglobulinemia
Bruton's X-linked agammaglobulinemia (XLA) is a genetic disease which is manifested by a serious immunodeficiency. It is the first known immunodeficiency with confirmed heredity. It was diagnosed in 1952 for the first time by doctor Ogden Bruton. [1]
Disease
Etiology and pathogenesis
Bruton’s agammaglobulinemia is caused by an abnormal product of a gene coding for Bruton’s tyrosine kinase (BTK). BTK belongs to the Tec family of non-receptor protein kinases notable for a pleckstrin homology domain. BTK is expressed in a great amount in B-lymphocyte precursors.
Under normal circumstances, during B-lymphocyte development IgH (BCR heavy chain) gene segments rearrange then the rearrangement takes place for the light chain. Disease causing BTK mutation aborts this process after rearrangement of IgH, light chains are not synthesized, immunoglobulins cannot be assembled which leads to apoptosis of B-cell precursors. The cause is most likely a malfunction of a signal path which transfers information from B-lymphocyte precursor receptors and in which BTK kinase is involved. [2]
The critical part on the long arm of X chromosome linked to this disease is Xq21.3-q22. It contains 19 exons 37,5 kb long. [3] The final protein is composed of 659 amino acids and of 5 domains: TH, SH3, SH2, PH and a kinase domain. Over 1 000 mutations causing XLA were identified in this gene. [4] In 40% of cases the defective allele is inherited, in 60% a spontaneous mutation arises. So it is an X-linked recessive hereditary disease. Due to that the disease usually occurs only in males, and females are healthy carriers. Prevalence in USA is 1 in 379,000 births in total and 1 in 190,000 births of male. [2]
BTK is mostly produced by precursors and immature cells, in mature lymphocytes the intracellular amount is decreased. In plasmatic cells it is not produced. Mutation in BTK causes a malfunction of other types of cells than B-lymphocytes, e.g. thrombocytes, macrophages and osteoclasts nevertheless in a smaller scale. After the activation of BCR, BTK is moved from the cytoplasm to the cell membrane where it is phosphorylated by Src family kinase. In cooperation with the adaptor B-cell linker protein (BLNK) it phosphorylates Cγ2 phospholipase which triggers another signal cascade. BTK interacts with TLR4, TLR6, TLR8, TLR9 and key proteins in signal paths of TLR like myeloid differentiation protein 88 (MyD88), MyD88 adapter-like protein (mal) , and interleukin-1 receptor (IL-1R)-associated kinase-1 (IRAK-1). BTK is important even for the congenital immunity. [5]
Clinical picture
Defective BTK has a consequence that a patient’s body cannot produce mature B-lymphocytes so it does not produce antibodies at all or at least in minimal scale. So it is not able to effectively defend against the infections.
The disease manifests itself after 6th month of age, at the time mother’s antibodies leave the child’s body. Typical are serious infections of breath paths and skin caused by commensal bacteria (S. Pneumoniae, H. Influenzae type B, S. Pyogenes, Pseudomonas) also usual are repetitive inflammation of the middle ear. The proneness to the enteroviruses (poliovirus, echovirus, coxsackievirus) is increased which is manifested in increased occurrence of meningitis and hepatitis. Approximately in 20% of patients arthritis is developed during the life most likely due to joint infections. [2]
Diagnostics and therapy
One of the criteria for diagnostics of XLA is less than 2% of CD19 B-cells in peripheral blood excluding other causes of hypoagammaglobulinemia, and at the same time, having one of these symptoms: repetitive bacterial infection, non-responsiveness to vaccines [6] or low level of serum antibodies. [7]
One of the most important parts of treatment of diagnosed patients is the prevention of bacterial infection contagion (increased hygiene, antibacterial lotions, …). It is recommended to cure any bacterial infection which patient gets by antibiotics to prevent serious or even deadly effects of these diseases. Then are these patients treated by intravenously given immunoglobulins. This treatment seems to be effective, the frequency of bacterial diseases is significantly decreased within the group of treated patients. Nonetheless the treatment is not able to fully compensate the function of antibodies as in a healthy individual. [2] In case of an infection, the level of serum antibodies does not elevate, and intravenously dosing immunoglobulin (IVIG) does not have the physiological proportions of the types of antibodies, IgG (95%) dominates and IgM and IgA are lacking. [8]
Another possible treatment of XLA is bone marrow transplantation. It fully heals the patient but there is a high chance for complications. The transplantation comes with the risk of graft-versus-host disease (GVHD). [9]
Bruton's tyrosine kinase
Function
Bruton's tyrosine kinase (BTK) is a non-receptor tyrosine kinase present in B lymphocytes, where it plays a key role in cell development, differentiation and signaling. For its proper function, one Zn2+ cofactor is needed. BTK acts as a platform to bring together various signaling proteins and is implicated in cytokine receptor signaling pathways. It is critical for the proper function of immune cells of both innate and adaptive immunity, as a component of the Toll-like receptors (TLR) pathway.
BTK also acts as a transcription regulator. Firstly, it is a part of the signaling pathway linking TLR8 and TLR9 (which causes BTK activation) to the NF-kappa-B complex. Inducing activity of this complex by its phosphorylation leads to significant changes of gene expression, since NF-kappa-B is involved in regulating the expression of hundreds of genes.
On top of that, BTK also phosphorylates transcription factor GTF2I in response to BCR. This regulator then translocates to the nucleus and binds regulatory enhancer elements to modulate gene expression. There is also a negative feedback mechanism to fine-tune BCR signaling. Activated PRKCB down-modulates BTK function via direct phosphorylation of BTK at Ser180, resulting in translocation of BTK back to the cytoplasmic fraction. [10]
|
There are many molecules identified as BTK activity inhibitors, for example PIN1, SH3BP5, and IBTK. Some of them are specific (LFM-13A), others cause a dramatic down-regulation of the kinase activity (CAV1). BTKs activity is also blocked by Dasatinib, a cancer drug acting as a non-specific tyrosine kinase inhibitor. [10]
It has been shown, that alternative splicing results in multiple transcript variants encoding different isoforms [11]
Protein domains
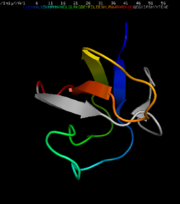
BTK consists of five functional domains. These are PH (Pleckstrin homology), TH (Tec homology), SH3, SH2 (Src homology) and C-terminal kinase domain. This composition is a typical feature of the Tec family of cytosolic protein tyrosine kinases, where BTK belongs. [12] Now, a brief characterisation of each domain follows:
PH domain
Tec family kinases are the only Tyrosine kinases, that contain a PH domain. Besides, PH domain is present in various proteins involved in signalisation, such as phospholipase C or GTPase activating proteins. There is a low sequence similarity between PH domains in various proteins, however they all share similar fold. With the length of 131 amino acids in BTK, it is the second largest domain. The N terminal part (namely the three conserved lysines) is responsible for binding phosphoinositides and leading them to its targeting to the plasma membrane, where it activates the NF-kappaB complex. The C terminus, together with the following TH domain plays a role in binding ßgamma subunits of heterotrimeric G proteins. [10] [13]
TH domain
The TH domain, an 80 amino acids long region preceding the SH3 domain, is unique for Tec family. It consists of N-terminal Btk motif followed by proline-rich region (PRR). In the Btk motif, there are some potentially metal binding histidine and cysteines residues. In addition, two 10 amino acid motifs in the PRR of Btk have been shown to interact with the SH3 domains of Fyn, Lyn and Hck proteins. The association of full length Src family kinases with Btk, though, has not been proven yet. [13]
SH3 domain
The SH3 domains are modules which bind polyproline stretches containing polypeptides and proteins. In many kinases, SH3 domains are in close proximity to SH2 ones, but in Tec family, the domains have only a few intervening residues.[13]
Kinase domain
The kinase domain is the largest one, forming more than 40% of BTK. It is found in the C terminus of the protein and is giving it its main function, being the only catalytic domain of BTK. The domain consists of two lobes. The N-terminal smaller upper lobe comprising mainly the five-stranded antiparallel β-sheet is primarily responsible for ATP binding. The ATP, donating the phosphate group, is bound to Lys430, located between the two lobes. The ATP binding residues are the most conserved sites in all protein kinases suggesting that both PSKs and PTKs functions similarly. The C-terminal larger lower lobe comprising mostly seven ɑ-helices is a major substrate-binding region. The active site is located at Asp 521. There are also multiple inhibitor binding sites in the kinase domain. The overall 3D fold of proteins containing related kinase domain is similar despite very low overall sequence similarities. [12][10] [13]
Regulation and localization
The regulation of the kinase is a highly complex process, where both the phosphorylation state and localization is important. In primary B lymphocytes, BTK is minely non-phosphorylated (and thus catalytically inactive). In this steady state, BTK is mostly localized in cytoplasm. Following B-cell receptor (BCR) engagement by antigen, it translocates to the plasma membrane through its PH domain and gets activated by phosphorylation. This phosphorylation occurs in the activation loop. The upper lobe translocates, when activated in the following way: In the inactive form, the upper part is twisted relative to the lower lobe. When the enzyme is activated, the upper lobe rotates to lock the ATP molecule between the two lobes of the domain. Active states structure is more loose, with essential MG2+ and ATP bonded. This is a common mechanism for both phosphoserine and phosphothreonine kinases. [12][10][13] It's also known, that a fraction of BTK also translocates between the cytoplasm and the nucleus. But there is no evidence that BTK itself binds directly to DNA. [10]
Mutations
Mutations in BTK Many mutations causing XLA were identified until today. It seems that most of them have evolved independently. They occur in all of the functional domains. Moreover, the distribution of mutations is quite uniform so that all the domains are affected almost equally. The kinase domain, for example, is comprising circa 40% of the length and accounts for about 45% of the mutations. Approximately one third of the mutations are of the missense type and the rest consist of insertions, deletions, and nonsense and splice-site mutations. [12] Only a tiny fraction of the mutations is described in more detail here:
PH domain
BTK is the only protein known where mutations in the PH domain have a phenotype. Mutations of BTK residues in PH domain close to the sites corresponding to the conserved lysines lead to the disease The substitution E41K was shown to increase phosphorylation of tyrosine residues and membrane targeting. Thus, the BTK phosphorylation might be linked to membrane interaction. The BTK PH domain specifically bound liposomes containing phosphatidylinositol-3,4,5-triphosphate (IP3). Most of the BTK PH domain mutations are concentrated in the binding site region where they could disturb interactions. The binding was shown to be abolished by the mutation R28Y, causing XLA. Moreover, residues K12 and S14, which mutations are also involved in XLA, and residue E41 involved in the gain-of-function mutation, might be also involved in phosphoinositide binding. The other mutations usually distort the folding of the domain. Y40 is one of the other sites, which are probably critical for normal function of the PH domain, maintaining its structural stability and participating on IP binding. The Y40N and Y40C mutations both cause a loss-of-function phenotype in XLA and seems to result in a significantly low affinity for IP4. [12][13]
The structural model of the PH domain , shows these residues, along with Y40, cluster on the same plane of the binding site. The side chain of Y40 is buried by the loop between β-strands 3 and 4, and the hydroxyl group of Y40 could potentially form a hydrogen bond with the backbone of G50. Mutations at this site will probably have structural consequences on the conformation of the loop and the phosphatidylinositol binding site and maybe of the whole domain. Thus, they are likely to be damaging for the normal function of BTK. [12]
Site-directed mutations of the polyproline II (PPII) helix forming proline residues in the PRRs of the TH domain of BTK abolish binding to SH3 domain. Mutations P189A and P192A are likely to alter the conformation such that the polyproline stretch can no longer be recognized. [13]
SH3 domain
In SH3 domain, aberrant splicing and skipping of exon 9 leads to an in-frame deletion of 21 residues containing the 14 C-terminal residues of the SH3 domain. These are forming the last three ß-strands. Even though the aberrant protein is stable and has full kinase activity in vitro, the patients do have the disease. The deletion of those ß-strands seems to distort the structure but the spacing between the termini in the mutant protein corresponds to the normal BTK SH3 domain. Thus the connection to the rest of the BTK is normal and the deletion causes no major changes in the overall protein fold. [13]
Kinase domain
Speaking about the kinase domain, there are several different types of missense mutations known, affecting structural, functional and interacting residues. The severe XLA mutations occur mainly in the ATP-binding region and the predicted substrate binding part. Some of them where found in other functionally or structurally important sites. Let the mutation A508D be one example of all. It introduces a charged residue in the hydrophobic core of the domain and, therefore, is likely to alter its conformation. Other important sites, where mutation leads to XLA (residues 502, 506, 508 and 509), are located in alpha helix E of the lower lobe. Each of these seem to be critical for maintaining the proper overall structure. [12] [13]
References
- ↑ Vu QV, Wada T, Le HT, Le HT, Van Nguyen AT, Osamu O, Yachie A, Nguyen SN. Clinical and mutational features of Vietnamese children with X-linked agammaglobulinemia. BMC Pediatr. 2014 May 28;14:129. doi: 10.1186/1471-2431-14-129. PMID:24885015 doi:http://dx.doi.org/10.1186/1471-2431-14-129
- ↑ 2.0 2.1 2.2 2.3 Taneja A, Chhabra A. Bruton Agammaglobulinemia PMID:28846295
- ↑ Zheng B, Zhang Y, Jin Y, Yu H. A novel Bruton's tyrosine kinase gene (BTK) missense mutation in a Chinese family with X-linked agammaglobulinemia. BMC Pediatr. 2014 Oct 15;14:265. doi: 10.1186/1471-2431-14-265. PMID:25316352 doi:http://dx.doi.org/10.1186/1471-2431-14-265
- ↑ Lee J, Rhee M, Min TK, Bang HI, Jang MA, Kang ES, Kim HJ, Yang HJ, Pyun BY. A novel BTK gene mutation, c.82delC (p.Arg28 Alafs(*)5), in a Korean family with X-linked agammaglobulinemia. Korean J Pediatr. 2016 Nov;59(Suppl 1):S49-S52. doi: 10.3345/kjp.2016.59.11.S49. , Epub 2016 Nov 30. PMID:28018445 doi:http://dx.doi.org/10.3345/kjp.2016.59.11.S49
- ↑ Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, Christensson B, Berglof A, Vihinen M, Nore BF, Smith CI. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009 Mar;228(1):58-73. doi: 10.1111/j.1600-065X.2008.00741.x. PMID:19290921 doi:http://dx.doi.org/10.1111/j.1600-065X.2008.00741.x
- ↑ Segundo GRS, Nguyen ATV, Thuc HT, Nguyen LNQ, Kobayashi RH, Le HT, Le HTM, Torgerson TR, Ochs HD. Dried Blood Spots, an Affordable Tool to Collect, Ship, and Sequence gDNA from Patients with an X-Linked Agammaglobulinemia Phenotype Residing in a Developing Country. Front Immunol. 2018 Feb 16;9:289. doi: 10.3389/fimmu.2018.00289. eCollection, 2018. PMID:29503650 doi:http://dx.doi.org/10.3389/fimmu.2018.00289
- ↑ Lim LM, Chang JM, Wang IF, Chang WC, Hwang DY, Chen HC. Atypical X-linked agammaglobulinaemia caused by a novel BTK mutation in a selective immunoglobulin M deficiency patient. BMC Pediatr. 2013 Sep 27;13:150. doi: 10.1186/1471-2431-13-150. PMID:24074005 doi:http://dx.doi.org/10.1186/1471-2431-13-150
- ↑ Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005 Oct;142(1):1-11. doi: 10.1111/j.1365-2249.2005.02834.x. PMID:16178850 doi:http://dx.doi.org/10.1111/j.1365-2249.2005.02834.x
- ↑ Howard V, Myers LA, Williams DA, Wheeler G, Turner EV, Cunningham JM, Conley ME. Stem cell transplants for patients with X-linked agammaglobulinemia. Clin Immunol. 2003 May;107(2):98-102. PMID:12763478
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 2019. UniProtKB - Q06187 (BTK_HUMAN). [Online] 2019. https://www.uniprot.org/uniprot/Q06187
- ↑ Smith, C.I.E. a Berglof, A. 2019. Homo sapiens Bruton tyrosine kinase (BTK), RefSeqGene (LRG_128) on chr - Nucleotide - NCBI. [Online] 2019. https://www.ncbi.nlm.nih.gov/nuccore/NG_009616.1
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Saha BK, Curtis SK, Vogler LB, Vihinen M. Molecular and structural characterization of five novel mutations in the Bruton's tyrosine kinase gene from patients with X-linked agammaglobulinemia. Mol Med. 1997 Jul;3(7):477-85. PMID:9260159
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 13.8 Vihinen M, Kwan SP, Lester T, Ochs HD, Resnick I, Valiaho J, Conley ME, Smith CI. Mutations of the human BTK gene coding for bruton tyrosine kinase in X-linked agammaglobulinemia. Hum Mutat. 1999;13(4):280-5. PMID:10220140 doi:<280::AID-HUMU3>3.0.CO;2-L 10.1002/(SICI)1098-1004(1999)13:4<280::AID-HUMU3>3.0.CO;2-L
Stewart, Donn M., Lan Tian, and David L. Nelson. "The clinical spectrum of Bruton’s agammaglobulinemia." Current allergy and asthma reports 1.6 (2001): 558-565. Vihinen, Mauno, Mattsson, Pekka T. and Smith, C. I. Edvard. 1997. BTK, THE TYROSINE KINASE AFFECTED IN X-LINKED AGAMMAGLOBULINEMIA. Frontiers in Bioscience 2, d27-42. [Online] 1997. https://www.bioscience.org/1997/v2/d/vihinen1/htmls/6.htm.
Credits
This article was created by Jan Keil, Barbora Kovandová, Adam Král and Linda Rendlová for Structural Biology of the Cell class, Charles University, summer 2019. (PyMol software used to create the images)