User:Christopher Koehn/sandbox 1
From Proteopedia
Contents |
Introduction to Sodium-Potassium-ATPases
The sodium-potassium-ATPase, also known as the Na-K pump or the sodium pump, is the protein responsible for the ATP-dependent, coupled transport of sodium and potassium ions across the plasma membrane. The Na-K pump is found on the surface of all animal cells and is a major force in maintaining the concentration gradients of these ions across the membrane [1]. These gradients provide energy for several cellular functions including control of membrane potential and cell size, pH homeostasis, and uptake of nutrients and water [2]. In each cycle of ATP hydrolysis, the protein transports three Na+ ions out of the cell and two K+ ions across the plasma membrane into the cell. This enzyme not only pumps ions against their gradients, but does so rather efficiently. Purified Na-K-ATPase has been shown to have turnover rates of 8,000 to 10,000 cycles per minute, though in cultured cells the turnover rate was decreased to between 1,500 and 5,000 cycles per minute [3]. The number of expressed Na/K pumps differs by cell type but is generally between 80,000 and 30 million [3]. In addition to its role as a transport protein, the sodium-potassium-pump has also been shown to act as a receptor for cardiotonic steroid signaling [4]. The sodium-potassium pump was first described in 1957 by Jens C. Skou and he was awarded the Nobel Prize in Chemistry in 1997 for this discovery[1].
Structure
|
()
The Na+-K+ pump is a P-type ATPase with a structure similar to the H+-K+-ATPase[2] and the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)[3] [1]. Overall, the structure of the sodium-potassium-pump is a transmembrane protein with three subunits labeled α, β, and FXYD[2].
α-Subunit
The α-subunit is the largest subunit and contains the binding sites for Na+, K+, and ATP. This subunit is composed of 10 transmembrane α-helices (M1-M10). These helices are centered around a three helix bundle formed by M4-M6[1]. The binding sites for K+ and Na+ are located within the transmembrane helices. Additionally, there are located on the cytoplasmic face of the membrane: the actuator domain (A), the nucleotide-binding domain (N), and the phosphorylation domain (P)[5]. There are 4 known isoforms of the α-subunit, but even the two most divergent isoforms share 78% sequence identity. The majority of structural diversity among the isoforms occurs at the N-terminus, the first extracellular loop, and the third cytosolic domain. This diversity can influence the rate ion transport and the ability to act as a signaling receptor [4].
β-Subunit
The β-subunit is a single spanning membrane protein with a transmembrane α-helix and a glycosylated extracellular domain [2]. This subunit uses a to bind to the M7 and M10 helices of the α-subunit within the lipid bilayer. These residues also make contact with a cholesterol molecule, the presence of which is necessary for ion transport to occur. Contact between the α and β subunits also occurs at various residues in the extracellular domains [5]. The β-subunit has important roles in targeting the polypeptide to the membrane and in providing stability. It plays a role in providing binding specificity for potassium ions [5].
FXYD Subunit
The FXYD subunit, sometimes known as the γ-subunit, is an accessory regulatory protein comprised of a transmembrane α-helix and an extracellular domain (which is not shown in this structure)[2]. Regulation of ion pumping action by FXYD has been shown to be tissue and isoform specific [2].
|
Mechanism of Na/K Pumping
The sodium-potassium pump transports cations across the membrane using what is the "alternative access" model, in which the protein alternates between two conformations, E1 and E2 [1]. The crystal structure of the E2 conformation in its phosphorylated state has been reported [2],[5]. No crystal structure is available for the E1 structure, but many aspects of it have been predicted based on homology to SERCA. An animation by Mark Hilge illustrating this mechanism can be found here[4]
E1 and E1-P
In the E1 state, ATP is bound at the N domain and high affinity Na+ binding sites are open to the cytosol [1]. Binding of three Na+ ions causes a conformational change that rotates the N domain so that the γ-phosphate of ATP is positioned near the phosphorylation site of the P domain. ATP is then cleaved and the γ-phosphate is transferred to [1]. The A domain then rotates around a horizontal axis large translation and a kink in the first transmembrane helix. This action closes the cytosolic gate of the protein, leaving the three Na+ molecules momentarily occluded in the transmembrane region of the protein [1]. ADP is also released at this time. This state is known as E1-P. Because E1-P is a high energy state, it rapidly relaxes to E2-P through a conformational change which kinks helices M5 and M7 and also opens the extracellular gate [1],[5].
E2-P
In the E2-P state, the affinity for Na+ ions is reduced, allowing them to dissociate into the extracellular environment. Next, extracellular K+ ions bind at two high affinity sites. These sites are believed to be composed of largely the same residues as two of the predicted Na+ sites. is composed of the carbonyl oxygen of Thr779; the side chain oxygens of Ser782, Asn783, and Asp811; and a water molecule fixed by interactions with Asp815 [5]. The kinks in M5 and M7 position Thr779 and Asn783 correctly for K+ binding at this site. Valence calculated for K+ binding at this site is 1.06, very close to the ideal value of 1.0. The valence for Na+ at this site in this state is only 0.62, explaining the lower affinity [5]. is 1.3 Å to the extracellular side of site I. This site is composed of the carbonyl oxygens of Val329, Ala330, and Val332; and the side chain oxygens of Asn783, Glu786, Asp811, and possibly Glu334 [5]. The valence calculated for K+ binding at this site is 0.67, which is lower than site I valence, but still higher than the Na+ binding valence of 0.54 [5]. In addition to the two extracellular K+ binding sites, there is also a located near the phosphorylation site (the phosphate group is represented by an MgF4 analog) [5]. Binding of this ion is important for the next conformational change.
E2
When both extracellular K+ cations are bound, a conformational change occurs which closes the extracellular gate, occluding the potassium cations [2]. Binding of the cytoplasmic K+ cation promotes helix-helix interactions between the A and P domains, triggering dephosphorylation [5]. The dephosphorylated protein is now in the E2 state and has an open intracellular ATP binding site. When ATP binds at this site, the protein reverts to the E1 conformation, the cytosolic gate opens, and the K+ cations dissociate from the binding sites which now have affinity for sodium cations [1].
Receptor Functions
|
Cardiotonic Glycoside Binding
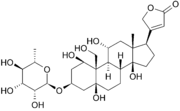
In addition to its role in ion pumping, the Na-K-ATPase can also serve to mediate cell signaling. This role is primarily fulfilled by acting as a receptor for cardiotonic glycosides, such as ouabain or digoxin [6]. These compounds bind to and inhibit the Na-K pump and both endogenous and exogenous examples are known [4]. Inhibition of the sodium-potassium pump causes an increase in intracellular Na+ concentrations, which in turn causes the Na+/Ca2+ exchanger to slow, ultimately leading higher intracellular Ca2+ concentration and stronger muscle contraction. Because of this effect, cardiotonic glycosides have been used as a treatment for congestive heart failure and supraventricular arrhythmias for over 200 years [6].
Ouabain has been shown to bind to the sodium-potassium pump in its E2-P state, just after potassium cations bind. The is located in the extracellular side of the transmembrane cleft, the same channel through which ions enter and leave the enzyme. The protein interacts with ouabain through over 30 residues and binds to all three components of the glycoside (lactone ring, steroid, and sugar). Ouabain binding is thought to inhibit the pumping action of the membrane by prohibiting the conformational changes that mark the transition to E2, such as the closure of the extracellular gate [6].
Protein-Protein Interactions
Direct Interaction with Src
In addition to the ion-concentration dependent effects described above, Na-K-ATPase receptor activation by cadiotonic glycoside binding can also transduce a signal through direct and indirect interactions with other proteins. One of the major types of proteins that interacts with the Na-K pump is the Src family of kinases. When the Na-K-ATPase is not activated by ouabain, an Src kinase binds to a cytosolic domain of the α subunit with both its SH2/SH3 domain and its kinase domain, creating a complex that has no kinase activity. Binding of ouabain causes the kinase domain of Src to dissociate from Na-K-ATPase, forming an active complex. By complexing with Src, Na-K-ATPase, which has no intrinsic kinase activity, can act similarly to other receptor tyrosine kinases [4].
Src-mediated Interactions
After receptor activation, Src phosphorylation has been shown to activate several proteins and is involved in cross-talk with other signaling cascades. One target of Src is the EGF receptor. Transactivation of this receptor by Src leads to activation of the Ras/Raf/MEK kinase cascade. Src also interacts with PI3K and PLC to initiate their associated pathways [4].
Interaction with Structural Proteins
In addition to signal transduction, the Na-K-ATPase can also interact with several scaffold/structural proteins. One of these proteins is caveolin-1, the hallmark protein of caveolae formation. Around 50% of sodium-potassium pump molecules have been shown to localize within caveolae [4]. This localization may be explained by the need of Na-K-ATPase for cholesterol, which is found in abundance in caveolae. Another scaffolding protein that associates with the sodium-potassium pump is ankyrin. Ankyrin uses the sodium-potassium pump as an anchor to bring the ER membrane protein IP3R, an IP3 gated Ca2+ channel, near the plasma membrane. This action brings together IP3R and PLC, its effector, to create what has been called a calcium signaling micro-domain [7].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Horisberger JD. Recent insights into the structure and mechanism of the sodium pump. Physiology (Bethesda). 2004 Dec;19:377-87. PMID:15546856 doi:19/6/377
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007 Dec 13;450(7172):1043-9. PMID:18075585 doi:10.1038/nature06419
- ↑ 3.0 3.1 Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007 Apr 6;282(14):10585-93. Epub 2007 Feb 12. PMID:17296611 doi:10.1074/jbc.M609181200
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys. 2006;46(3):303-16. PMID:17272855
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 Shinoda T, Ogawa H, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature. 2009 May 21;459(7245):446-50. PMID:19458722 doi:10.1038/nature07939
- ↑ 6.0 6.1 6.2 Ogawa H, Shinoda T, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13742-7. Epub 2009 Aug 3. PMID:19666591
- ↑ Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology (Bethesda). 2008 Aug;23:205-11. PMID:18697994 doi:23/4/205