User:Elizabeth Tomao/sandbox1 alphaB-crystallin
From Proteopedia
|
Contents |
The Mysterious Roles of αβ-crystallin
Introduction
The protein αβ-crystallin is small heat shock protein (HSP) that is part of crystallin family as well as the α cystistallin subfamily. It is closely related to αA- crystalin, there are a few major differences between the two proteins. αA crystallin is mostly found in the ocular lens, whereas αβ-crystallin can be found in the ocular lens but also in other tissues such as the lungs, kidneys, brain, and cardiac muscles. Heat shock proteins may also be known as stress proteins because they are activated under conditions of stress; such as heat, change in pH, amount of oxygen and other materials such as metals. When activated these heat shock proteins act as chaperones, by assisting in the folding and folding and to prevent the aggregation or misfolding of other proteins. αβ-crystallin accumulates in tissues when they are exposed to any type stress, therefore it is common in a tissue that is being affected by a disease. It has been found in the following tissues brain, heart, kidneys, skeletal muscle, placenta, and skeletal muscle and associated with such diseases as Parkinson ’s disease, Alzheimer’s Disease, Alexander’s Disease, Multiple Sclerosis, Cancers, and Heart Disease. This small protein acts as a form of defense by preventing the disease from attacking certain cells.
Structure and Function
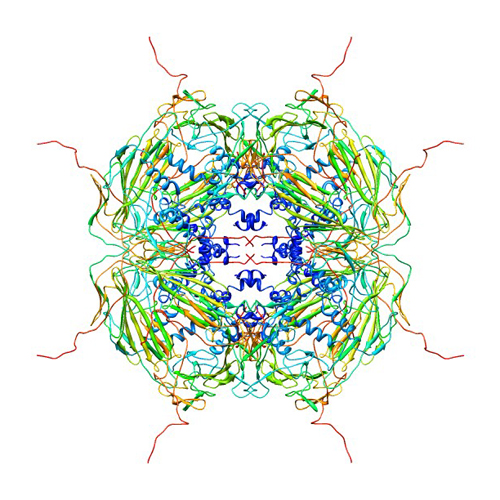
αβ-crystallin is small containing 20kDa that is normally formed into oligomers that are around 600kDa. There are three sections of the αβ-crystallin, the α- crystallin domain ( which is ninety residues long and made of β sheets folded into a sandwich like formation with β8-β-9β-3(β-2) and β4-β4- β6+7. (green Link) On either side of the ACD are the N and C- terminals. The N- terminal (Green Link) is sixty residues long and the C- terminal twenty five residues long (green link). It has been found it is these terminals that are essential for the proper functioning of the αβ-crystallins. Studies conducted by removing parts of the N and C- terminals shoed as decrease in chaperone activity each with their own role in the proper functioning of the αβ-crystallin. The N-Terminal contains a hydrophobic interactive sequence (41STSLSPFYLRPPSFLRAP58) (green link) which is extremely important in substrate selection. It is the nature of this sequence that recognizes and selects substrates to protect against aggregation based on the amount of protein unfolding. The C- Terminal on the other hand contains a polar interactive sequence (155PERTIPITREE165)( Green Link) which imperative for conserving the solubility of the unfolded proteins. These regions have been proven to be conserved among different species. A study conducted in The Netherlands worked with mice and found the same sequence in hamsters. This is the closest, αβ-crystallin is extremely conservative, this proteins rarely undergoes mutations. Though these proteins are do not change very often, when assembled into oligomers they are extremely polydiverse, this makes it very hard to study this group of proteins. This continuous reconfirmation is said to be a result of the residues on HRI(green link) because of its lack of structure.
The sections of αβ-crystallin that are integral to the performance of the protein the terminals merely explain how it is that these little proteins have the ability to prevent apoptosis or mitosis of cancer cells. Much of the function of these proteins has to do with where and how they bind to other molecules, which occurs at numerous binding sites. Many studies have been carried out to determine the binding sites and it wasn’t until recently that scientists unveiled some information about this process. In a pin array study conducted in 2006 by the Mason Eye Institute, where results indicated that binding sites can be found on the sequences 9-20, 43-48, 113-120, 131-138, 141-148. In addition to effective bonding sites there are parts of this molecule that are essential for their chaperone function. Chaperone proteins help proteins assemble or disassemble. These special chaperone sequences include the mentioned C- Terminal and N-Terminal sites along with 75FSVNLDVK82 (β3) (green link), 131LTITSSLS138 (β8) (green link), 141GVLTVNGP149 (β9) ( green Link). It is along these sites where the αβ-crystallin oligomer can dissociate and bind to the unfolded protein and a complex that prevents the prtein from further unfolding. (Picture)
The αβ-crystallin family thrives upon the presence of stress and therefore functions best when exposed to varying levels of pH, temperature, certain metal ions, and ATP. A study was conducted in 2006 to tests the effects of the mentioned stresses. The results showed that chaperone activity increased under high temperatures, acidic conditions, whereas the Ca+ and ATP had no affect on the activity of αβ-crystallin. These conditions mimic the environments that are present when certain disorders occur.
Association with Diseases
Cancer
Associations with certain diseases The small heat shock protein has been found to reduce the rate of mitosis is Cancer cells by acting as a cofactor to FBX4 which is a part of a ligase hat regulates the G1 and S phase of the cell cycle.
MS and Neurodegenerative Diseases
αβ-crystallin can be found in large numbers along the lesions on the brain of MS patients. Studies performed on mice have shown that with the help of αβ-crystallin, the effects of multiple sclerosis can be reversed. This has lead to much research to find a therapeutic use for the protein to help with MS, where αβ-crystallin is administered to prompt T-cell tolerance. αβ-crystallin can also accumulate in patients with other neurodegenerative disorders such as Alzheimers and Parkinson ’s.
Heart Disease
When Ischemic injury occurs it causes small heat protein localization, where αβ-crystallin along with other heat shock proteins phosphorylate and prevent apoptosis, and maintain stability and flexibility among the stressed heart muscles. This occurs in saccromere proteins actin, titin, and the intermediate filaments
Current Uses & Future Studies
Along the β6+7, two anti- parallel monomers form an extended beta sheet to form the Anti- parallel which serves as a binding site, where elements such as Cu+ bond and increase the chaperone activity of αβ-crystallin. This process of adding metals such as copper acts as way to test αβ-crystallins’ ability to contribute to protein stability and oxidative stress resistance, which may in the future be used to help treat patients.
Refernces
1. Andi Mainz, Benjamin Bardiaux, Frank Kuppler, Gerd Multhaup, Isabella C. Felli, Roberta Pierattelli, and Bernd Reif Structural and Mechanistic Implications of Metal Binding in the Small Heat-shock Protein αB-crystallin
2. J. Biol. Chem. 2012 287: 1128-1138. First Published on November 15, 2011, doi:10.1074/jbc.M111.309047 Y Quax-Jeuken, W Quax, G van Rens, P M Khan, and H Bloemendal Complete structure of the alpha B-crystallin gene: conservation of the exon-intron distribution in the two nonlinked alpha-crystallin genes PNAS 1985 82 (17) 5819-5823
3. 3. U Jakob, M Gaestel, K Engel, and J Buchner Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993 268: 1517-20.
4. Cite error: Invalid <ref> tag;
refs with no content must have a name PMID: 20535035 Cite error: Invalid <ref> tag;
refs with no content must have a name
5. Dana A Haley, Joseph Horwitz, Phoebe L Stewart, The small heat-shock protein, αb-crystallin, has a variable quaternary structure, Journal of Molecular Biology, Volume 277, Issue 1, 20 March 1998, Pages 27-35, ISSN 0022-2836, 10.1006/jmbi.1997.1611. (http://www.sciencedirect.com/science/article/pii/S0022283697916119)
6. Cite error: Invalid <ref> tag;
refs with no content must have a name PMID:17105203 Cite error: Invalid <ref> tag;
refs with no content must have a name
7. Cite error: Invalid <ref> tag;
refs with no content must have a name PMCID: PMC2242417 Cite error: Invalid <ref> tag;
refs with no content must have a name
8. Andi Mainz, Benjamin Bardiaux, Frank Kuppler, Gerd Multhaup, Isabella C. Felli, Roberta Pierattelli, and Bernd Reif Structural and Mechanistic Implications of Metal Binding in the Small Heat-shock Protein αB-crystallin J. Biol. Chem. 2012 287: 1128-1138. First Published on November 15, 2011, doi:10.1074/jbc.M111.309047
9. Lingyun Liu, Joy G. Ghosh, John I. Clark, Shaoyi Jiang, Studies of αB crystallin subunit dynamics by surface plasmon resonance, Analytical Biochemistry, Volume 350, Issue 2, 15 March 2006, Pages 186-195, ISSN 0003-2697, 10.1016/j.ab.2005.12.019. (http://www.sciencedirect.com/science/article/pii/S0003269705008924)