Introduction
GLP-1 is an incretin that functions to lower blood glucose levels upon binding to GLP-1R, a G-protein coupled receptor. The precursor of GLP-1, proglucagon, was first discovered in 1983. GLP-1 was then isolated from proglucagon in 1986. GLP-1 is released from L cells in the intestine and its receptor can be found in the pancreas, GI tract, brain, and cardiovascular system. [1] The structure was determined using electron microscopy. Upon binding to the receptor, GLP-1 stimulates insulin secretion from pancreatic 𝛽 cells, making GLP-1R agonists a viable treatment for Type 2 Diabetes Mellitus. Further, GLP-1 promotes glycolysis by recruiting GLUT2 transporters and stimulating the enzyme glucokinase. The first GLP-1R agonist for diabetes was created in 2005, and several more have been developed since. GLP-1 has various other biological roles, including regulation of bone metabolism, memory, and cardiac function. [2] Because GLP-1 stimulates insulin release, inhibits glucagon secretion, and decreases food intake through promoting satiation, GLP-1R agonists are also currently being used as weight loss drugs. [1]
Structure
The GLP-1 receptor is made up of : extracellular, transmembrane, and intracellular. It has four main components that make up its structure: a , a with 𝛼, 𝛽, and 𝛾 subunits, and a . The nanobody and SCV fragment 16 are not part of GLP-1R’s actual structure; they were put in place to help identify and stabilize the rest of the receptor during electron microscopy studies. [3] GLP-1 itself is a helical incretin that can exist as either a non-amidated, 31 residue peptide or a C-terminus amidated, 30 residue peptide. While both forms of the peptide exert similar effects on the body through GPCR signaling, the amidated form is more prevalent and exists in roughly a 3:1 ratio naturally. [2]
Ligand Binding Site
Binding Interactions of GLP-1
GLP-1 engages in several with the GLP-1R. The N-terminus of GLP-1 binds within the transmembrane region of GLP-1R, and the C-terminus of GLP-1 binds to the N-terminus of GLP-1R. Upon GLP-1 binding, a signal cascade is triggered beginning with an elevation in cAMP levels. Increased intracellular cAMP levels activate PKA and EPAC2 leading to several downstream signaling effects including increased ATP production, increased insulin production and secretion, and membrane depolarization. [1] Beginning at the N-terminus of GLP-1, forms a salt bridge with GLP-1R R190 and a hydrogen bond with GLP-1R Y152. Next, hydrogen bonds to GLP-1R K197. Towards the middle of the peptide chain, hydrogen bonds with GLP-1R R299 and GLP-1 E21 hydrogen bonds with GLP-1R Y205. At the of GLP-1, hydrogen bonds with GLP-1R Q210. Right above this, there is a pi stacking interaction between GLP-1 F28 and W31 with GLP-1R W214. [4]
Binding Interactions of Tirzepatide
Tirzepatide is an imbalanced GLP-1R/GIP-R coagonist. Tirzepatide has an equal affinity for the GIP-R as native GIP does, but it has a lower affinity for GLP-1R than native GLP-1 does. [5] Because of this, there is a biased signaling that results from Tirzepatide binding. This manifests as greater cAMP generation and lower beta-arrestin recruitment resulting in a lesser degree of GLP-1R internalization. Consequently, more GLP-1R is exposed on the surface of cells.
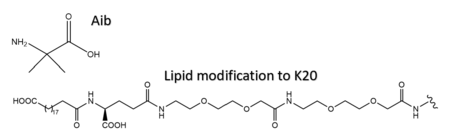
Structure of 2-Aminoisobutyric acid and the C20 fatty diacid moiety. Tirzepatide is modified with Aib at position 2 and 13 and the fatty diacid at K20.
Several residues have been selectively and intentionally modified during Tirzepatide drug design. , aminoisobutyric acid, is located at positions 2 and 13 with the purpose of preventing DPP-4 cleavage and inactivation. K20 of Tirzepatide has a lipid modification, specifically a C20 fatty diacid moiety, that serves to enhance binding to the protein carrier albumin and increase the half-life of the drug in the body. [5]
Similarly to GLP-1 forming several ligand binding interactions with GLP-1R, Tirzepatide also forms many key with GLP-1R. Beginning at the N-terminus of Tirzepatide (Tirz), forms a salt bridge with GLP-1R R190 and a hydrogen bond with GLP-1R Y152. Additionally, Tirz T7 hydrogen bonds with GLP-1R K197. These interactions are nearly identical to the interactions GLP-1 E9 and T13 make with the receptor. Towards the middle of the peptide, forms a hydrogen bond with Y205, yet another similar interaction to GLP-1 binding. [4] Looking at the sequence comparison of GLP-1 and Tirzepatide, GLP-1 E21 is located at the same position as Tirz D15.
Comparison of Binding Interactions
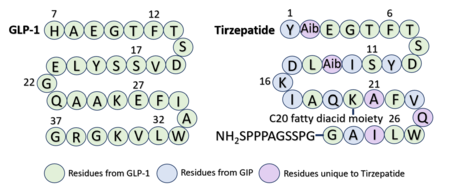
Sequence alignment of GLP-1 and Tirzepatide.
Through an , it is visible that GLP-1 and Tirzepatide bind similarly but not identically to the receptor. Some interactions seen in GLP-1 bound to GLP-1R are preserved with Tirzepatide, while others are altered. For example, the first residue of GLP-1 is a , while the first residue of Tirzepatide is a . [5] The identity of this first residue can either favor or prevent specific GLP-1R interactions. With GLP-1 H7, and E373 are in close enough proximity to form a salt bridge. Additionally, GLP-1R W306 and D372 are positioned ideally for hydrogen bond formation. However, with Tirzepatide bound, Tirz Y1 causes greater steric clashing, pushing the and other residues further apart so they are unable to interact. [5]
Another example of a difference in receptor conformation can be seen with GLP-1R R299. With GLP-1 bound, faces the peptide and is able to hydrogen bond with either GLP-1 S17 or E21. When Tirzepatide is bound, flips away from the peptide and can no longer hydrogen bond with any Tirzepatide residues. [5] Additionally, differential binding of GLP-1 and Tirzepatide modifies how the transmembrane domain interacts with the G-alpha subunit to initiate a signal cascade. For example, when GLP-1 is bound to the receptor, is able to participate in a pi stacking interaction with G-alpha F376. When Tirzepatide is bound, is oriented facing away from the G-alpha subunit. [4]
Referencing the sequence alignment, GLP-1 F28 and W31 are in the same position as Tirzepatide F22 and W25, indicating that the phenylalanine and tryptophan residues are conserved among the two sequences. As discussed previously in the GLP-1 binding interactions subsection, and W31 participate in a pi stacking interaction with GLP-1R W214. Similarly, and W25 are also able to interact aromatically with GLP-1R W214.
Medical Relevance
There have been several drugs designed to target the GLP-1 receptor. In 2014, Trulicity (dulaglutide) was FDA approved for weekly injections as treatment for Type 2 Diabetes. In 2017, Ozempic (semaglutide) was FDA approved as both a weekly injection or a daily oral pill to regulate blood glucose. The GLP-1R/GIP-R coagonist Mounjaro (tirzepatide), FDA approved in 2022, has shown a significant superiority over GLP-1 agonists alone as both a treatment for Type 2 Diabetes and as a weight loss drug. [6] Promising initial clinical trial results are now being demonstrated for retatrutide: a GLP-1R/GIP-R/glucagon (GCG) receptor triple hormone agonist. Data from phase 2 clinical trials reveal an average of 24.2% weight loss over the course of 48 weeks, suggesting retatrutide as a viable future treatment for obesity. [7]


