User:Luca Paulino Otvos/Sandbox 1
From Proteopedia
|
Contents |
Function
The Protein Tyrosine Phosphatase localized to the Mitochondrion 1 (PTPMT1) is a dual specificity phosphatases (DUSP) that catalyzes the dephosphorylation of phosphothreonine, phosphoserine and/or phosphotyrosine residues in lipid or protein substrates. Its name came from the fact that it was the first phosphatase identified exclusively in mitochondria [1]. PTPMT1 is a phosphatase directed to the mitochondria by an N-terminal sequence and is found anchored in the inner mitochondrial membrane with its phosphatase domain facing the matrix [2].
PTPMT1 is also a phosphatidylinositol phosphate (PIP) phosphatase, showing specific activity in vitro to different phosphatidylinositol phosphate molecules, such as PI(5)P [1][3], PI(3,4)P2 and PI(3,5)P2 [3]. Due to its mitochondrial location, PI(3,5)P2 appears to be the best PIP substrate for PTPMT1 [3].
Biological roles
One of the best known functions of this phosphatase is its participation in the biosynthetic pathway of cardiolipin [4], a phospholipid that makes up the inner mitochondrial membrane and is essential for metabolic functions, crest structuring and mitochondrial dynamics [5]. In this process, PTPMT1 is responsible for the dephosphorylation step of phosphatidylglycerolphosphate (PGP) forming phosphatidylglycerol (PG), which is the substrate of cardiolipin synthase.
PTPMT1 is also involved in glucose homeostasis and insulin secretion in pancreatic β cells. The silencing of this phosphatase in the INS-1 832/13 cell line results in a significant increase in ATP production and insulin secretion [2]. These results were further explained, based on the discovery that PTPMT1 is able to dephosphorylate and, consequently, inactivate succinate dehydrogenase, an enzyme that participates in the reaction of succinate to fumarate, and is probably important in the regulation of this enzyme in the Krebs cycle [6].
Structural highlights and Activity
PTPMT1 is a small protein (210 amino acids in Homo sapiens and 261 amino acids in Mus musculus) characterized by a catalytic domain smaller than that of other PTPs (characteristic of DUSPs) and by the ability to dephosphorylate phosphothreonine, phosphoserine and/or phosphotyrosine residues, in addition to having activity on substrates proteins and lipids [7] [8] [9].
The is composed of six α-helix and five β strands. Furthermore, its protein structure also presents loops regions, some of which are important in protein phosphatase activity.
That protein features three essential for its catalytic function: the P loop, D loop, and the EEYE loop [10] [11].
The P loop corresponds to the catalytic site of protein tyrosine phosphatases (PTPs). It consists of a catalytic cysteine, followed by any five amino acids and an arginine (CX5R motif). Another conserved region important for the function of this phosphatase is the WPD loop (or D loop in DUSPs), which contains the aspartate necessary for the dephosphorylation reaction to proceed [11]. In the initial phase of this process, catalytic cysteine nucleophilically attacks the phosphate of the target residue and aspartate acts as a general acid, donating a proton to the reaction [12]. The conserved arginine of the P loop is extremely relevant during this step, as it guarantees the stability of the penta-coordinated transition state, in which the phosphate makes five bonds, one with catalytic cysteine, another with substrate residue and the too much with its oxygen atoms [13]. As a result, an intermediate phosphoenzyme is generated, whose cysteine carries the phosphate group removed from the substrate. Aspartate then acts again, but this time as a general base, removing a proton from a water molecule [12]. Finally, this water molecule nucleophilically attacks the catalytic cysteine phosphate, breaking the bond between them and restoring the phosphatase conformation [11] [14].
A third region identified in DUSPs and other PTPs is the , a variable regulatory portion contained between the (or β7 depending on the protein phosphatase) [15]. It is in this region where the EEYE loop characteristic of PTPMT1 and other DUSPs is found [11]. Xiao et al. (2011) demonstrated the importance of the EEYE loop for the PTPMT1 phosphatase function, based on mutations in the glutamate residues of this loop. Alterations in E141A and E144A resulted in a marked decrease in PTPMT1 activity.
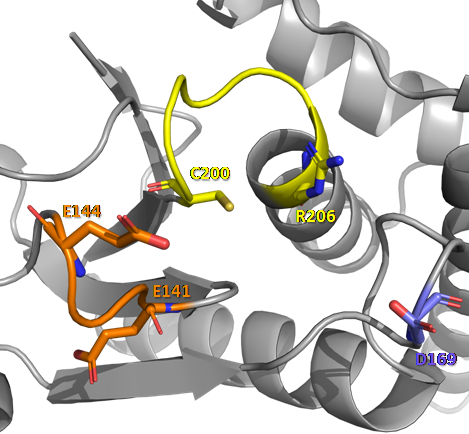
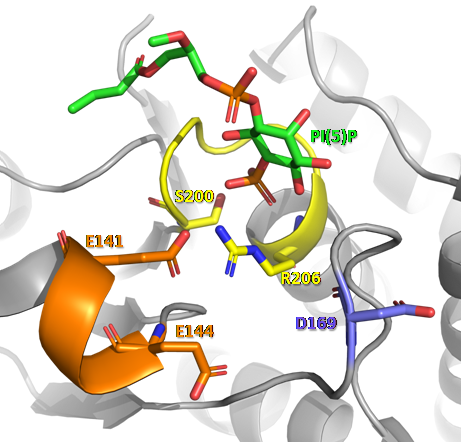
Through the analysis of the crystal structure of mouse PTPMT1 (169-261) in its apo form (PDB 3RGO) and linked to PI(5)P (PDB ), it was observed that the EEYE loop undergoes a major conformational change and these glutamates are responsible for ensuring the proper conformation of the conserved arginine of the catalytic motif.
In the figure above, we can observe the conformational changes that occur in the structural regions that participate in the catalytic activity of PTPMT1, when this phosphatase protein interacts with a PI(5)P molecule. In D loop, the conserved aspartate undergoes an approximation towards the active site, where the dephosphorylation reaction takes place. In P loop, there is rotation and approximation of residues of the CX5R catalytic motif towards the center of the active site. In the EEYE loop, the most evident conformation change occurs, with a refolding of the chain, forming a small helix in the most N-terminal portion of this loop, and the approximation of glutamate residues (E141 and E144) to the active site. These modifications allow the formation of non-covalent interactions (hydrogen bonds, salt bridges and interactions through water molecules)[11] between the molecules that participate in the reaction, enabling the catalysis mechanism described above.
Disease
Despite little knowledge about diseases related to the mitochondrial protein phosphatase PTPMT1, studies have demonstrated the importance of this protein in the process of differentiation of early embryonic stem cell (ESC) lines [3] [16]. The need for PTPMT1 in ESC would be related to the rapid metabolic transition that occurs in the differentiation process, in which stem cells start to present a high biogenesis of mitochondria and increased aerobic metabolism. According to Shen et al., 2011 and Yu et al., 2013, the depletion of PTPMT1 causes an increase in the amounts of phosphatidylinositol (3,5) bis-phosphate (PIP) in the mitochondria, which would lead to decreased oxygen consumption capacity and increased fragmentation of mitochondria, due to deficiency in membrane traffic and mitochondrial dynamics. As a result, the demands on embryonic stem cells are not met and a cell signaling cascade leads to interruption of the cell differentiation program.
Another important point to be highlighted is the participation of PTPMT1 in the biosynthetic pathway of cardiolipin, a structural component of the mitochondrial inner membrane, which is also related to mitochondrial dynamics [5] and whose absence can lead to mitochondrial dysfunction, which causes cellular consequences associated with different types of human diseases such as neurogeneration and muscle atrophy [3].
Relevance
PTPMT1 phosphatase (or DUSP23) is highly conserved among animals, plants, protists and bacteria [2], which suggests the great importance of the functions performed by it within mitochondria.
An interesting aspect is the possibility of using PTPMT1 as a therapeutic target in the treatment of type II diabetes , since the knockdown of the expression of this phosphatase caused an increase in cellular ATP levels and insulin secretion by pancreatic β cells [2]. Thus, the application of inhibitors of this mitochondrial protein phosphatase in pancreatic islets may be a promising antidiabetic method [9] [17].
The importance of PTPMT1 in cancer cells is also being studied [18]. Niemi et al., found that the silencing of this protein phosphatase by RNAi is capable of inducing the death of cancer cells, through a decrease in cardiolipin levels, causing mitochondrial dysfunction, and an increase in cellular ATP levels in a medium containing glucose. These changes cause a metabolic crisis in cancer cells, leading to cell death. In this sense, PTPMT1 becomes a possible target to promote the sensitization of cancer cells, allowing greater efficiency of chemotherapy methods.
References
- ↑ 1.0 1.1 Pagliarini DJ, Worby CA, Dixon JE. A PTEN-like phosphatase with a novel substrate specificity. J Biol Chem. 2004 Sep 10;279(37):38590-6. doi: 10.1074/jbc.M404959200. Epub 2004 , Jul 9. PMID:15247229 doi:http://dx.doi.org/10.1074/jbc.M404959200
- ↑ 2.0 2.1 2.2 2.3 Pagliarini, D.J., Wiley, S.E., Kimple, M.E., Dixon, J.R., Kelly, P., Worby, C.A., Casey, P.J., Dixon, J.E., 2005. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol. Cell 19, 197–207. https://doi.org/10.1016/j.molcel.2005.06.008
- ↑ 3.0 3.1 3.2 3.3 3.4 Shen J, Liu X, Yu WM, Liu J, Nibbelink MG, Guo C, Finkel T, Qu CK. A critical role of mitochondrial phosphatase Ptpmt1 in embryogenesis reveals a mitochondrial metabolic stress-induced differentiation checkpoint in embryonic stem cells. Mol Cell Biol. 2011 Dec;31(24):4902-16. doi: 10.1128/MCB.05629-11. Epub 2011 Oct , 10. PMID:21986498 doi:http://dx.doi.org/10.1128/MCB.05629-11
- ↑ Zhang J, Guan Z, Murphy AN, Wiley SE, Perkins GA, Worby CA, Engel JL, Heacock P, Nguyen OK, Wang JH, Raetz CR, Dowhan W, Dixon JE. Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab. 2011 Jun 8;13(6):690-700. doi: 10.1016/j.cmet.2011.04.007. PMID:21641550 doi:http://dx.doi.org/10.1016/j.cmet.2011.04.007
- ↑ 5.0 5.1 Lu YW, Claypool SM. Disorders of phospholipid metabolism: an emerging class of mitochondrial disease due to defects in nuclear genes. Front Genet. 2015 Feb 3;6:3. doi: 10.3389/fgene.2015.00003. eCollection 2015. PMID:25691889 doi:http://dx.doi.org/10.3389/fgene.2015.00003
- ↑ Nath AK, Ryu JH, Jin YN, Roberts LD, Dejam A, Gerszten RE, Peterson RT. PTPMT1 Inhibition Lowers Glucose through Succinate Dehydrogenase Phosphorylation. Cell Rep. 2015 Feb 10;10(5):694-701. doi: 10.1016/j.celrep.2015.01.010. Epub 2015, Feb 5. PMID:25660020 doi:http://dx.doi.org/10.1016/j.celrep.2015.01.010
- ↑ Alonso A, Pulido R. The extended human PTPome: a growing tyrosine phosphatase family. FEBS J. 2016 Apr;283(8):1404-29. doi: 10.1111/febs.13600. Epub 2015 Dec 16. PMID:26573778 doi:http://dx.doi.org/10.1111/febs.13600
- ↑ Lee H, Yi JS, Lawan A, Min K, Bennett AM. Mining the function of protein tyrosine phosphatases in health and disease. Semin Cell Dev Biol. 2015 Jan;37:66-72. doi: 10.1016/j.semcdb.2014.09.021. Epub, 2014 Sep 26. PMID:25263013 doi:http://dx.doi.org/10.1016/j.semcdb.2014.09.021
- ↑ 9.0 9.1 Park H, Kim SY, Kyung A, Yoon TS, Ryu SE, Jeong DG. Structure-based virtual screening approach to the discovery of novel PTPMT1 phosphatase inhibitors. Bioorg Med Chem Lett. 2012 Jan 15;22(2):1271-5. doi: 10.1016/j.bmcl.2011.10.083. , Epub 2011 Nov 3. PMID:22115589 doi:http://dx.doi.org/10.1016/j.bmcl.2011.10.083
- ↑ Jeong DG, Wei CH, Ku B, Jeon TJ, Chien PN, Kim JK, Park SY, Hwang HS, Ryu SY, Park H, Kim DS, Kim SJ, Ryu SE. The family-wide structure and function of human dual-specificity protein phosphatases. Acta Crystallogr D Biol Crystallogr. 2014 Feb;70(Pt 2):421-35. doi:, 10.1107/S1399004713029866. Epub 2014 Jan 29. PMID:24531476 doi:http://dx.doi.org/10.1107/S1399004713029866
- ↑ 11.0 11.1 11.2 11.3 11.4 Xiao J, Engel JL, Zhang J, Chen MJ, Manning G, Dixon JE. Structural and functional analysis of PTPMT1, a phosphatase required for cardiolipin synthesis. Proc Natl Acad Sci U S A. 2011 Jul 5. PMID:21730175 doi:10.1073/pnas.1109290108
- ↑ 12.0 12.1 Denu JM, Zhou G, Guo Y, Dixon JE. The catalytic role of aspartic acid-92 in a human dual-specific protein-tyrosine-phosphatase. Biochemistry. 1995 Mar 14;34(10):3396-403. doi: 10.1021/bi00010a031. PMID:7880835 doi:http://dx.doi.org/10.1021/bi00010a031
- ↑ Zhang ZY, Wang Y, Wu L, Fauman EB, Stuckey JA, Schubert HL, Saper MA, Dixon JE. The Cys(X)5Arg catalytic motif in phosphoester hydrolysis. Biochemistry. 1994 Dec 27;33(51):15266-70. doi: 10.1021/bi00255a007. PMID:7803389 doi:http://dx.doi.org/10.1021/bi00255a007
- ↑ Alonso A, Nunes-Xavier CE, Bayon Y, Pulido R. The Extended Family of Protein Tyrosine Phosphatases. Methods Mol Biol. 2016;1447:1-23. doi: 10.1007/978-1-4939-3746-2_1. PMID:27514797 doi:http://dx.doi.org/10.1007/978-1-4939-3746-2_1
- ↑ Yuvaniyama, J., Denu, J.M., Dixon, J.E., Saper, M.A., 1996. Crystal structure of the dual specificity protein phosphatase VHR. Science 272, 1328–1331. https://doi.org/10.1126/science.272.5266.1328
- ↑ Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, Finkel T, Broxmeyer HE, Qu CK. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013 Jan 3;12(1):62-74. doi: 10.1016/j.stem.2012.11.022. PMID:23290137 doi:http://dx.doi.org/10.1016/j.stem.2012.11.022
- ↑ Doughty-Shenton D, Joseph JD, Zhang J, Pagliarini DJ, Kim Y, Lu D, Dixon JE, Casey PJ. Pharmacological targeting of the mitochondrial phosphatase PTPMT1. J Pharmacol Exp Ther. 2010 May;333(2):584-92. doi: 10.1124/jpet.109.163329. Epub , 2010 Feb 18. PMID:20167843 doi:http://dx.doi.org/10.1124/jpet.109.163329
- ↑ Niemi NM, Lanning NJ, Westrate LM, MacKeigan JP. Downregulation of the mitochondrial phosphatase PTPMT1 is sufficient to promote cancer cell death. PLoS One. 2013;8(1):e53803. doi: 10.1371/journal.pone.0053803. Epub 2013 Jan 10. PMID:23326511 doi:http://dx.doi.org/10.1371/journal.pone.0053803