User:Martha Blakely/Sandbox 1
From Proteopedia
|
(MAO-B) is a mitochondrial outer-membrane flavoenzyme. It is one of two isozymes (MOA-A is the other), which catalyzes the oxidative deamination of amine neurotransmitters, including serotonin, dopamine, and epinephrine. Selectivity is as follows: MAO-A oxidizes serotonin and epinephrine, MAO-B oxidizes benzylamine and phenylethylamine, and both enzymes oxidize dopamine. The flavin-dependent enzymes use O2 as an electron acceptor in the catalytic pathway which includes hydrogen peroxide, H2O2, as a product. Increased H2O2 levels promote apoptotic signaling of cells. Thus, researchers have found that increased levels of MAO-B, whose levels in the brain increase at least 3-fold on aging, are related to decreased levels of neuronal cells. The cells in the brain particularly targeted are dopamine-producing cells, which results in the development of Parkinson’s disease. A popular example related to the development of Parkinsonian symptoms based on MAO-B levels is the oxidation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to 1-methyl-4-phenylpyridinium (MPP+) which destroys glial cells. The inhibition of MAO-B prevents against this cell death behavior.
Inhibitors of MAO-B such as deprenyl and rasagiline have been developed in order to protect the brain from neurological disorders. Deprenyl is used in L-dopa therapy which acts as treatment and protection for Parkinson's and pre-Parkinson's patients.
Contents |
Structure
|
Structurally, MAO-B is a dimer with each monomer composed of 520 amino acids. The sequence forms a helix on each monomer that is responsible for the attachment to the outer-membrane of mitochondria. This α-helix resides in the lipid bilayer. In addition to the C-terminal helices, other hydrophobic side chains such as also contribute to attachment.
The active site of MAO-B is a flat hydrophobic cavity that is separated into two parts, the entrance cavity and the substrate cavity. The dual nature of the active site allows for binding of differently sized substrates. Some are large enough to fill both cavities, some only fill the substrate cavity, and others are too small to fit either. The wide range of substrate size can partially be contributed to a boundary between the two cavities. Four residues () form this boundary between cavities. The positioning of these four residues affects which substrates and inhibitors are able to bind to MAO-B. Ile199 is a gating residue that can be in the “open” or “closed” positions.
Entrance into the connected cavities is determined by the movement of a loop of amino acids () located at the protein surface near the membrane attachment. This loop prevents solvent from entering the active site. The loop’s location is near the region of the protein that binds with the mitochondrial outer-membrane. This proximity implies that passage into the active site can only occur near the membrane.
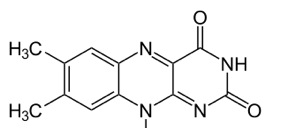 Flavin moiety of FAD cofactor with an empty N5 bond.
Flavin moiety of FAD cofactor with an empty N5 bond.
Each monomer has its own covalently bound. The FAD molecule is situated on the opposite end of the active site away from the entrance into the cavity. It aids in binding substrates and inhibitors which seem to be situated between Tyr398 and Tyr435 when bonded to the N5 position of the FAD cofactor (generally called “flavin moiety”). Inhibitors and substrates must maneuver past these tyrosine residues, which contain phenol side chains, in order to bond to the N5 position of the FAD cofactor. These three together, the flavin, , form an aromatic cage.
The implications of these residues are important to know for their own effects on substrate binding. These important residues also show that further knowledge of the three-dimensional structure of MAO-B is important in the development of enzyme inhibitors.
Comparative to MAO-A
Both are flavoenzymes found attached to the outer-membrane of mitochondria. The amino acid sequences of the two are about 70% identical and are encoded by separate genes on the X-chromosome. Both catalyze the oxidative deamination of amine neurotransmitters, but the enzyme selectivity of the neurotransmitters varies. In their crystalline form, MAO-A is a monomer, whereas MAO-B is a dimer. Depression and mood swings are related to higher levels of MAO-A, rather than diseases like Parkinson’s and Alzheimer’s that are associated with higher levels of MAO-B. Serotonin, a neurotransmitter related to mood elevation, is specific to MAO-A. The residues that correspond to binding are also different between these two enzymes.
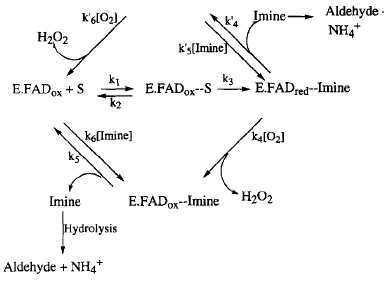
Catalytic Pathway
The oxidative deamination of amine neurotransmitters is catalyzed by both monoamine oxidases. The image above shows the two generally proposed and accepted catalytic pathways for this oxidation; the lower loop is the case for most substrates. In this loop, the enzyme-imine compound (E.FADred--Imine) reacts with oxygen (O2) to create H2O2 and the oxidized enzyme-imine compound (E.FADox). The imine is then reduced to the ammonium ion (NH4+) and its respective aldehyde. The cycle goes around the loop another time when an amine substrate (S) binds to the active site within the enzyme (E.FADox + S).
Hydrogen peroxide is shown as a product in this catalytic pathway. Due to hydrogen peroxide's association with health problems such as Parkinson's disease and Alzheimer's disease, MAO inhibitors have been created with the intention of neuroprotective functions. The inhibitors must be bound to the active site of the enzyme, which means that knowledge of enzyme binding during the catalytic pathway with neurotransmitters is important for the development of these inhibitors.
Inhibition
Both reversible and irreversible inhibitors have been developed for MAO-B. Irreversible inhibitors form a covalent bond to the FAD cofactor in the enzyme active site, permanently deactivating it. Concentrations of the inhibitor and the possible substrates determine whether a reversible inhibitor binds to the enzyme. Rasagiline and deprenyl (also seleginine) are both FDA-approved irreversible inhibitors used for the inhibition of MAO-B. Rasagiline is much more selective for MAO-B than MAO-A. Under normal administration, deprenyl is selective for MAO-B, but when deprenyl is administered in large amounts, its selectivity for MAO-B decreases. In this case, it also inhibits MAO-A. Safinamide is an example of a reversible inhibitor for MAO-B.
References
- Binda, Newton-Vinson, Hubalek, Edmondson, and Mattevi (2002) "Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders", Nature Structural Biology 9: 22-26.
- Edmondson, Binda, Wang, Upadhyay, Mattevi (2009) "Molecular and Mechanistic Poperties of the Membrane-Bound Mitochondrial Monoamine Oxidases", Biochemistry 48: 4220-4230.
- Youdim, Edmondson, Tipton (2006) "The therapeutic potential of monoamine oxidase inhibitors", Nature Reviews 7:295-309.