Function
Zepatier is a combination drug combating genotypes 1 and 4 of chronic hepatitis C viral (HCV) infections in adults. Elbasvir inhibits NS5A, a non-enzymatic protein essential for viral replication and assembly of hepatitis C whose exact mechanism of action is unknown. Elbasvir prevents viral production at the early stage of assembly in turn preventing spread of the virus.
Grazoprevir is a macrocyclic compound that is able to reversibly bind to the protease, NS3/4A. NS3/4A is a serine protease also essential for viral replication. It is responsible for cleavage and processing of the HCV polyprotein. It is hypothesized that the protease is utilized to circumvent immune response at the primary stages of infection [1].
Structure and Mechanism of Elbasvir
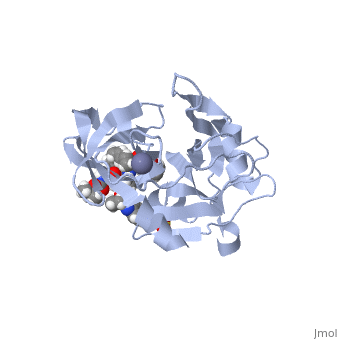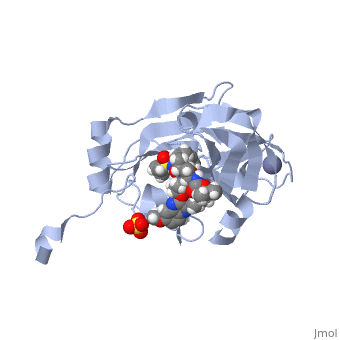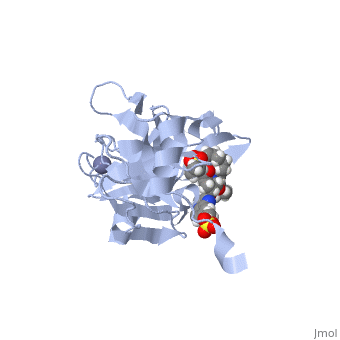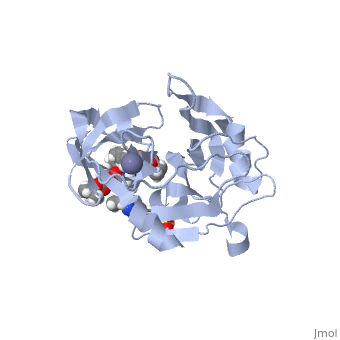
, MK-8742,a tetra-cyclic indole based NS5A inhibitor, was experimentally obtained through the modification of MK-4882. MK-4882 was one of the first clinical candidate drugs as an NS5A inhibitor. Although initially effective, viral breakthrough was a concern which led to continue development of compounds that would be effective against multiple genotypes as well as NS5A mutations. Through efficacy studies of infected chimpanzees, Elbasvir was discovered as a diastereomer of the known compound, MK-4882 [2]. Elbasvir has a structural formula of C49H55N9O7 and a molecular weight of 882.035 g/mol. It is a highly flexible protein with 13 rotatable bonds. It has four hydrogen bond donors and nine hydrogen bond acceptors [3].
Although the actual mechanism of Elbasvir as a NS5A inhibitor is unknown, several hypothetical mechanisms have been proposed. The NS5A protein is a critical protein for both DNA replication and assembly in the hepatitis C virus. With critical roles in both processes, NS5A is an excellent source as a target for inhibitors. NS5A is naturally located in the endoplasmic reticulum of the cell. When inhibited, the protein is redistributed from the ER to lipid droplets. If the cellular location changed, NS5A would be unable to aid in viral replication, illustrating a form of protein inhibition that Elbasvir could cause. Another potential mechanism of NS5A inhibitors is the altering of the phosphorylation of the NS5A protein [4]. The function of the NS5A protein is highly dependent on the phosphorylation, both basal phosphorylation and hyperphosphorylation [5]. Since the phosphorylation of the NS5A protein is critical for protein function, altering the levels of phosphorylation could impact the activity of the protein.
Structure and Mechanism of Grazoprevir
stops the hepatitis C virus by inhibiting the NS3/4A protease, which is an enzyme responsible for the cleavage of the viral polyprotein. The molecular formula is C38H50N6O9S and a molecular weight of 766.911 g/mol [6]. Cleavage of this polyprotein facilitates viral assembly and maturation of the virus. The NS3/4A protease is a protein complex in which the active site consists of a catalytic triad of amino acids S139, H57 and D81, and an activating cofactor which assists in, and is required for the activity of the protease. Grazoprevir has been proven to be more effective than similar protease inhibitors due to its two-way approach to inhibition. NS3/4A protease cleaves both the viral polyprotein of the Hep C virus and host factors involved in the immune response, such as TRIF and MAVS. Grazoprevir is unique in regard to its P4 cap, and P2 to P4 macrocyclic restriction, which interacts with the catalytic triad of the NS3/4A protease in its own distinct conformation. In this conformation, the P2 region is comprised of the Histidine-57 and Aspartate-81 residues.
Disease in Humans
Hepatitis C, hereafter known as HCV, is a viral infection that causes inflammation of the liver and decreasing liver functioning. First isolated by Michael Houghton in 1989, hepatitis C was discovered to encode a single polyprotein of 3,000 amino acids later cleaved into 10 polypeptides. Polypeptides produced include p7 ion channel proteins, the core protein, glycoproteins envelope 1 (E1) and envelope 2 (E2), and nonstructural proteins NS1, NS2, NS3, NS4A, NS5A, and NS5B. The 5’ untranslated region of HCV is not capped, allowing it to fold into a complex secondary RNA structure hereby creating an internal ribosome entry site that is able to direct ribosomal subunits and cellular factors, and as a result, translation. Development of cell culture based model systems have allowed for the study of the HCV lifecycle [7].
HCV is transmitted exclusively through blood whereupon the virus travels to the hepatocytes of the liver to undergo rapid replication. This results in the inflammation of the liver, leading to decreased function and/or liver failure. HCV induces an immune response within the infected individual which commonly fails to restrict the development of chronicity.
While six distinct genotype of HCV were discovered, genotypes 1 is the most prevalent amongst populations and the most difficult to treat. Increased resistance of the virus at the M28, Q30, L31, or Y93 amino acids results in decreased effectiveness of pharmaceuticals against genotype 1. Genotype 1 results from the infection of human lymphoid cells by the virus. Genotype 4 has recently increased in prevalence, resulting in attempts to decrease transmission.
Zepatier is a combination drug designed for the treatment of individuals with end stage renal disease on dialysis, typically genotype 1 infected patients. The drug is also utilized to treat genotype 4 patients when other methods of treatment prove ineffective [8].