User:Michael O'Shaughnessy/ TS
From Proteopedia
Contents |
Overview
This page is a work in progress during the spring 2022 semester.
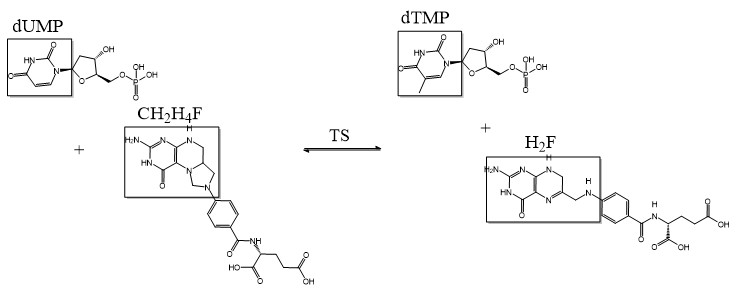
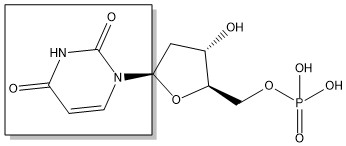
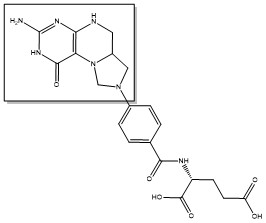
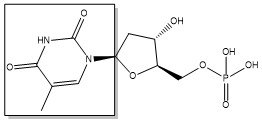
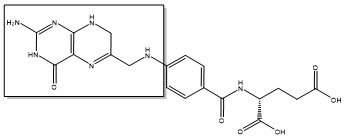
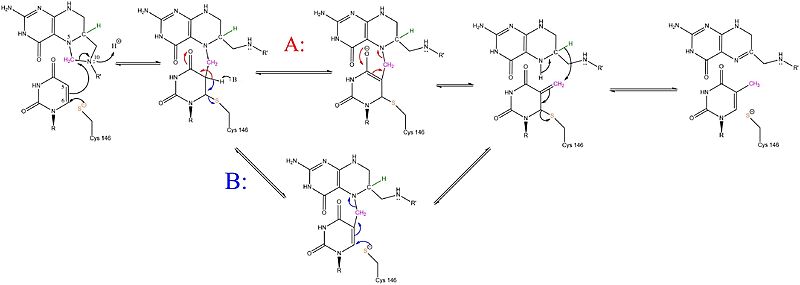
The enzyme Thymidylate Synthase (TS) is an important enzyme in one-carbon metabolism[1]. TS catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP (a precursor to Thymine) in humans. [1]
2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH2H4F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H2F)
Disease
Relevance
Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH2H4F. Inhibition of TS halts the production of dTMP and, indirectly, 2'-deoxythymidine-5'-triphosphate (dTTP). Both dTMP and dTTP are essential building blocks for DNA synthesis and their absence halts the ability of cells to replicate their genetic information. This is especially effective in cancer cells that rapidly divide and require large amounts of dTMP and dTTP. [2]
| |||||||||||
References
- ↑ Kholodar SA, Finer-Moore JS, Swiderek K, Arafet K, Moliner V, Stroud RM, Kohen A. Caught in Action: X-ray Structure of Thymidylate Synthase with Noncovalent Intermediate Analog. Biochemistry. 2021 Apr 8. doi: 10.1021/acs.biochem.1c00063. PMID:33829766 doi:http://dx.doi.org/10.1021/acs.biochem.1c00063
- ↑ Costi MP, Ferrari S, Venturelli A, Calo S, Tondi D, Barlocco D. Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem. 2005;12(19):2241-58. doi: 10.2174/0929867054864868. PMID:16178783 doi:http://dx.doi.org/10.2174/0929867054864868
- ↑ Fritz TA, Tondi D, Finer-Moore JS, Costi MP, Stroud RM. Predicting and harnessing protein flexibility in the design of species-specific inhibitors of thymidylate synthase. Chem Biol. 2001 Oct;8(10):981-95. PMID:11590022