Background
The ABCG2 multidrug transporter is a membrane protein from the ATP-Binding Cassette (ABC) transporter family, specifically the G-subfamily. Also known as the breast cancer resistance protein (BCRP), ABCG2 has physiological roles in various tissue cells including the mammary gland and the blood-brain, blood-testis, and maternal-fetal barriers.[1] ABCG2 protects cells by exporting xenobiotic molecules out of the cell using ATP hydrolysis. ABCG2 also affects the pharmacokinetics of many drugs and contributes to multidrug resistance.[2] ABCG2 belongs to the family of 48 transporter proteins called ATP-binding cassette transporters (ABC transporters). A high percentage of the ABC family transporters (19 of the 48) transport chemotherapeutic agents out of cells, making expression levels of ABC transporters a major indicator of cancer treatment prognosis.[3]
Using cryoelectron microscopy (cryo-EM), the two cavity substrate transport structure (; ), inward facing nucleotide binding domain (), and condensed extracellular loop 3 () structure of ABCG2 have been elucidated. These structures also illustrated the transporter cycle of ABCG2, the binding locations for inhibitors, and the link between cancer and the ABC transporter family.[1][2][4]
Structural highlights
Overall Structure
ABCG2 is a homodimer with each monomer containing two domains, the nucleotide binding domain and the transmembrane domain , which are fused together as a single peptide chain.
[1] The NBD binds and processes ATP and is located inside of the cell where it is exposed to the cytosol. The TMD is responsible for binding and transporting substrates, is embedded in the cell membrane, extends into the extracellular region (Figure 1).
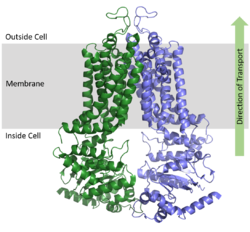
Figure 1. Orientation of ABCG2 in relation to the cell membrane.
(5NJ3) ATP Bound and Unbound Conformations
As an ABC Transporter, ABCG2 exhibits ATPase activity and uses the energy of ATP hydrolysis to facilitate transport. After substrates bind in the TMD, one molecule of (2 molecules of ATP total) causing a conformational change of the overall structure from an to an . with various residues and a magnesium ion in the which is bordered with Walker A and B motifs. One molecule of ATP is hydrolyzed to transport substrates across the cell membrane while the second molecule of ATP is hydrolyzed to reset the transporter to its inward-facing conformation.[3]
When ATP binds, α-helices in the NBD approximately 35° relative to the . This shift in the NBD causes slight shifts of α-helices in the TMD; these helices are relative to the . The overall shift from inward-facing to outward-facing promotes the transport of substrates through the transporter.[2]
The NBDs in ABCG2 remain in contact with one another even without a bound substrate, providing greater substrate specificity as the entrance to the transporter is not as globular as other ABC transporters like ABCB1 or ABCC1. The entrance from the cytoplasm to the transporter is lined by hydrophobic residues in both monomers.
Cavities and Leucine Plug
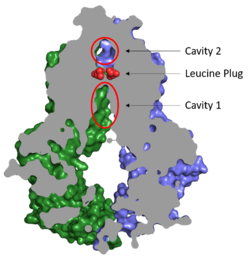
Figure 2. Locations of Cavities 1 and 2 and the Leucine Plug in ABCG2. The protein is in the inward-facing conformation with Cavity 1 open to the cytosol for substrate recruitment, the Leucine Plug intact, and Cavity 2 completely occluded.
(5NJ3)Substrates are transported through ABCG2 via two cavities separated by a leucine plug (Figure 2). acts as a multidrug binding pocket and is formed by helices at the interface of the monomers in the TMD. When ATP is not bound to the NBDs, Cavity 1 is in order to recruit substrates for transport. Cavity 1 is and full of nonpolar, hydrophobic residues and, as a result, prefers nonpolar, hydrophobic substrates, particularly flat, polycyclic molecules. Substrates, such as estrone sulfate, with residues from each subunit in Cavity 1.[1]
After substrates bind in Cavity 1, ATP binds each NBD leading to the transporter shifting from inward-facing to outward-facing. The outward-facing conformation results in the in the TMD in which the cavity is no longer . This collapse forces the substrate to move forward to Cavity 2 as there is no longer room in Cavity 1 to accommodate substrates.[2] , which is occluded when the protein in is the inward-facing conformation, is now open to the extracellular space and able to release the substrate. Cavity 2 contains a less hydrophobic environment and, as a result, substrates are released due to hydrophobic mismatch.[1] in the external loops near the exit of Cavity 2 also help promote substrate release.[2] Once Cavity 2 is empty, the protein reverts to the inward-facing conformation via hydrolysis of ATP.
Cavities 1 and 2 are separated by a which likely acts as a substrate check-point during transport; changes to either of these leucine residues have exhibited an increase in transport and a decrease in substrate specificity.[2] After the substrate binds Cavity 1 and ATP molecules bind each NBD, the to allow the substrate to enter Cavity 2. Once the substrate enters Cavity 2, the plug is able to reform and promote substrate release and conversion to the inward-facing conformation.
Structural Elucidation of ABCG2
Binding of two antigen binding fragments () were required for stabilization of ABCG2 for high resolution cryo-EM.[1][2] 5D3 Fab the two domains together preventing movement of the transporter from inward to outward facing. Fab binds at a 35 degree angle relative to the membrane plane which stops the 40 degree transition of the TMD from closed to open. Interestingly, complete arrest of ABCG2 in its inward facing state only requires one Fab bound but in experiments, two Fabs bound, each to both domains.[1][2]
Disease
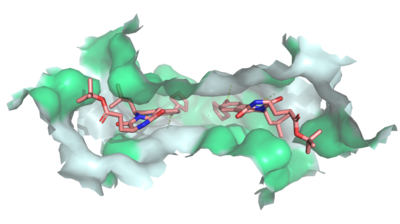
Figure 3: MZ29 bound to cavity 1 of ABCG2
(6FFC). Two MZ29 are shown in sticks and are colored by element. Hydrophobic interactions between the surface of cavity 1 and MZ29 are shown in green.
Dysfunctions in ABCG2 are linked to hyperuricemia which can lead to gout, kidney disease, and hypertension, all of which are thought to be the result of impaired transport of uric acid. Additionally, the expression of ABCG2 has been found to correlate with a poor prognosis and treatment outcome of various cancers including breast, ovarian, and lung.[4] Several mutations also decrease transporter activity.[1][2][3] The most detrimental of these is a point mutation of to Gln 211, which completely abolished the ATPase activity of the transporter. Another point mutation occurs at Gln141 (), which when mutated to lysine causes gout by distorting ABCG2's tertiary structure. Changes at shift the substrate specificity of ABCG2 by allosteric effects as this residue is distantly located from the binding pocket.
Cancer
ABCG2 hinders cancer treatment by contributing to multidrug resistance in tumor cells. ABCG2 exports xenobiotics, including vital anti-cancer drugs, which results in the inability to treat cancer cells. Cancer patients often show high levels of expression of multiple ABC transporters. For example, acute myeloid leukemia (AML) has an increased expression of ABCB1, ABCG1, and ABCG2 while childhood AML shows an increased expression in ABCA3, ABCB1, ABCC3, and ABCG2.[5][6] Additionally, pancreatic cancer has shown an upregulation of ABCB4, ABCB11, ABCC1, ABCC3, ABCC5, ABCC10, and ABCG2.[7]
The substrate specificity among ABC transporters varies, so this protein family can collectively export a wide variety of substrates and, ultimately, a wide variety of anticancer drugs (Figures 3 and 4). ABCG2 has been known to export anticancer drugs such methotrexate, mitoxantrone, topotecan, irinotecan, and flavopiridol[8]. Due to the high expression of multiple ABC transporters in cancer cells, simultaneous treatment of multiple transporters would likely be necessary for successful cancer treatment.
Inhibitors
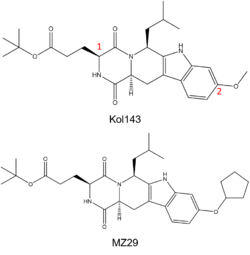
Figure 4. (Top) Known ABCG2 Inhibitor Kol143; Red numbers indicate points of alternation in derivatives of inhibitor. (Bottom) Derivative of Kol143, MZ29.
Due to the potential for ABCG2 inhibition to aid in cancer treatment, efforts have been made to develop specific inhibitors of ABCG2 and other ABC transporters. The ABC transporter ABCB1, also known as multidrug resistance 1 (MDR1), was a therapeutic target in previous studies which produced three generations of MDR1 inhibitors, such as verapamil, valspodar, and zosuquidar; however, many of these inhibitors had neurotoxic effects that discouraged their use in cancer treatment.[9][10][11]
While ABC transporter inhibition was dismissed after failed clinical trials, interest in revisiting ABC inhibition has reemerged due to new developments made in recent years.[3] For instance, Kol143 (Figure 4) is a compound derived from fungal toxin fumitremorgin C (FTC), a selective inhibitor of ABCG2 which exhibits undesirable neurotoxic effects.[12] Kol143 was found to be less toxic and more potent than FTC; however, this inhibitor is nonselective toward ABCG2.[13] Various inhibitors were derived from Kol143 with changes made at positions 1 and 2 in Figure 3, which are carbons 3 and 9 respectively. Modifications at these positions improved the inhibitory capacities of various Kol143 derivatives, including the derivative MZ29 which stabilized the inactive inward facing conformation (Figures 3 and 4).[4]
ABCG2 inhibitors, such as ()(Figure 3), bind Cavity 1 and act as competitive inhibitors against ABCG2 substrates by binding with a higher affinity toward the transporter than the natural substrates. Depending on the size of the inhibitor, one or two molecules can accommodate binding to the cavity and form within the binding site.[4] Many inhibitors are too big to be transported via the leucine plug resulting in the "clogging" of the transporter. With inhibitors acting as wedges, ABCG2 is locked in the inward-facing conformation and unable to transport molecules out of the cell.[2]




