Introduction
Azurin is a bacterial protein that has been extensively studied by bioinorganic and biophysical chemists as a prototype of a Type 1 or "blue" copper protein. It contains a single copper ion that can be in the Cu+ or Cu2+ or the Cu state. The intensely blue color is due to a charge transfer transition from the cysteine thiolate ligand to the Cu in the Cu2+ state. It functions as an electron transfer mediator. The electron transfer reactivity of azurin has been extensively studied, including studies of its reactivity with natural and artificial partners, and intramolecular electron transfer from intrinsic and covalently attached electron transfer partners. The latter studies have been instrumental in defining and evaluating the factors influencing electron transfer reactivity through proteins. These factors include the electron transfer distance, the structure of the intervening peptide medium, the thermodynamic driving force, and the structure of the donor and acceptor. These studies have been instrumental in the iterative testing and advancing of electron transfer theory.
One series of studies, delineated here, involves measurement of the rate constant for electron transfer from a disulfide radical, produced by pulse radiolysis, to the Cu2+ ion. This reaction can be made to occur because of particular structural features of azurin, the Cu2+ site is relatively buried and at the opposite end of the protein from the only disulfide, which is exposed to solvent and electron transfer reagents.
Structure
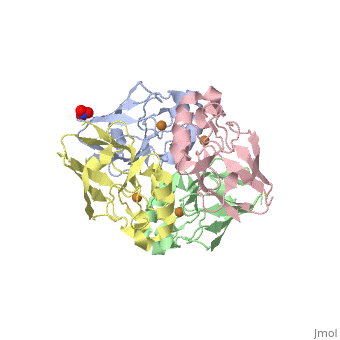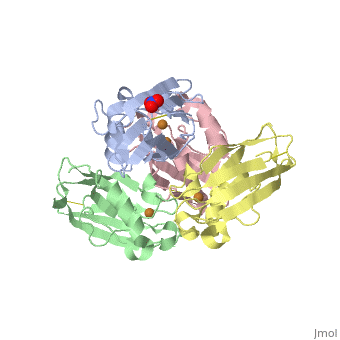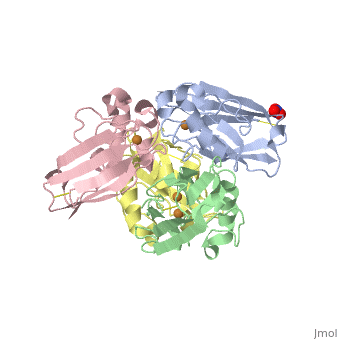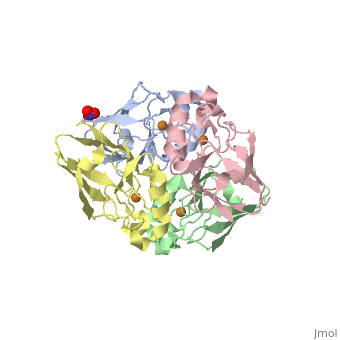
Azurin has 128 amino acids and a beta barrel structure, as exemplified by the structure of the Pseudomonas aeruginosa protein (4AZUA, chain A). The is shown colored by the secondary structure assignment (pink for alpha helix, purple for a 310 helix, yellow for beta strands, blue for beta turns, and white for other structures). The copper atom is at the top of this standard orientation. The primary ligands to the copper atom are the thiolate of Cys112, and ND His117 and His 46, forming a trigonal planar arrangement. In addition there are two weaker ligands, the S of Met121 and the carbonyl O of Gly45, occupying axial positions to give an approximately trigonal bipyramidal
.
Exposure of the disulfide and the copper site
The studies discussed here involve the intramolecular electron transfer between a one electron reduced disulfide radical and the oxidized copper ion. In order to measure the rate constant for this process, the disulfide radical must be produced rapidly by a strong reductant in a bimolecular reaction. This bimoleclar reaction must reduce the disulfide preferentially over the Cu2+ site. Azurin shows this preferential reactivity due to the lack of exposure of the copper site, with only part of the edge of the His117 exposed coupled with high exposure of the disulfide Cys3-Cys26.
The reducing agent typically used is the CO2- radical, an especially strong reducing agent produced by pulse radiolysis of formate containing solutions.
Electron transfer path
Electron transfer is slowed by distance between the donor and acceptor, but this can be partially ameliorated by an appropriately constructed pathway of covalent bonds. Thus the distance between the electron donor and acceptor can be analyzed in terms of pathways involving covalent bonds, hydrogen bonds (less effective), and through-space jumps (even less effective). One pathway between the disulfide and copper involves a covalent path from SG of Cys 3, through the backbone Ser 4, Val 5, Asp 6, Ile 7, Gln 8, Gly 9 and Asn 10, then through a hydrogen bond from the O of Asn 10 to the proton on NE2 (Ne) of the ligand His46. The second path branches through a hydrogen bond from the carbonyl O of Cys 3 to the peptide N of Thr 30, then through the backbone of Val 31 and then via a through-space jump from the side chain CG of Val 31 to CG of the sidechain of Trp 48, then through the side chain and backbone of Trp 48 and Val 49, followed by a hydrogen bond from the backbone N of Val 49 to to carbonyl O of Phe 111 and then to the Cu via the ligand Cys 112. The orbital coupling provide by this path is sensitive to the distance of the through-space jump, and thus is influenced by the mobility of the structure.