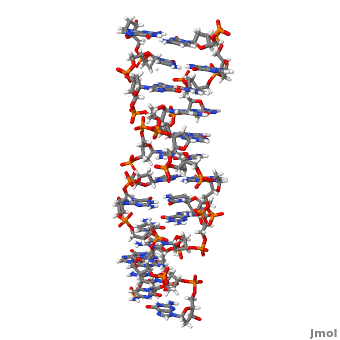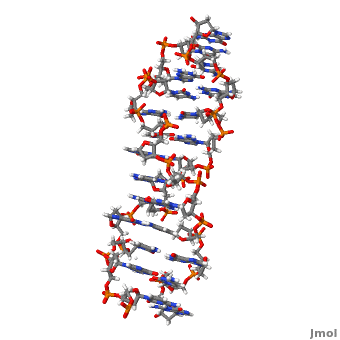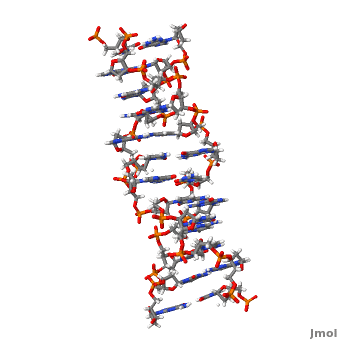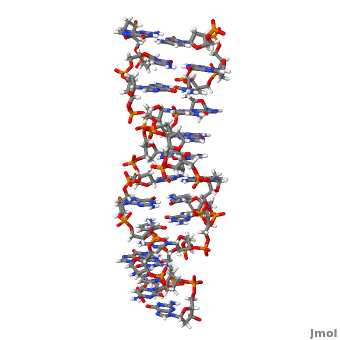
Z-DNA is a form of DNA that has a different structure from the more common form.It is a left-handed double helix wherein the sugar-phosphate backbone has a zigzag pattern due to the alternate stacking of bases in anti-conformation and syn conformation. In Z-DNA only a minor groove is present and the major groove is absent. The residues that allow sequence-specific recognition of Z-DNA are present on the convex outer surface.[1] This DNA form is thought to play a role in the regulation of gene expression, DNA processing events and/or genetic instability.[2]
See also Z-DNA model tour and B-DNA tour.
Structure
Z-DNA (, PDB entry 2acj) can form in vitro from B-DNA by raising negative super helical stress or under low salt conditions when deoxycytosine is 5-methylated. The formation of Z-DNA in vivo is an energy requiring process. It forms behind a RNA polymerase moving through a DNA double helix during transcription and is subsequently stabilized due to the generation of negative supercoils. Z-DNA is the first single crystal X-ray structure of a DNA fragment. It was crystallized as a self complementary DNA hexamer d(CG)3 by Andrew Wang, Alexander Rich and their co-workers at MIT in 1979. [1][2]
Whenever B-DNA transforms into Z-DNA two form. The crystal structure of these junctions revealed, and at the junction. A crucial finding from this structure is that a right handed DNA can transform to a left handed DNA or vice versa by the disruption and extrusion of a base pair. It has also been suggested that the extruded base pairs at B-Z DNA junction may be sites for DNA modification.[3]
Z-DNA binding proteins
Double Stranded RNA adenosine deaminase 1, ADAR1
ADAR1 (, 1qbj) belongs to the family of deaminases that modify double stranded mRNA by catalyzing the conversion of adenine to inosine which is then translated to guanosine. It is a complex protein with two Z-DNA binding motifs called and Z-beta.[2] ADAR1 also has three copies of double-stranded RNA binding motif (DRBM) and a catalytic domain related to E.coli cytidine deaminase. The binding motif Z-alpha belongs to winged-helix-turn-helix family of proteins. It consists of a which has two alpha helices ( and ) connected by a short strand of amino acids and a . The beta sheet constrains the fold by contacting the residues between alpha-2 and alpha-3.
The contact surface between consists of residues from the helix alpha-3 and COOH-terminal beta hairpin. Hydrogen bonding is present between Lys169, Lys 170, Asn173, Arg174 and Tyr177 in the helix alpha-3 and . Lys169, Asn173, Arg174, Trp195 make water mediated phosphate contacts with Z-DNA. In addition Thr191 and Arg174 of G2 and G6 on Z-DNA. An important interaction is the between aromatic ring of Tyr177 and the carbon 8 of G4. This is unique to Z-DNA as the interaction requires the base to be in syn conformation. Pro192, Pro 193 form another set of with Z-DNA where the pyrrolidine rings bond with the sugar-phosphate backbone from phosphate 2 to phosphate 3. Pro192 is conserved in Z-alpha and its homologues and forms a cis peptide bond which positions beta loop against the Z-DNA surface.[4]
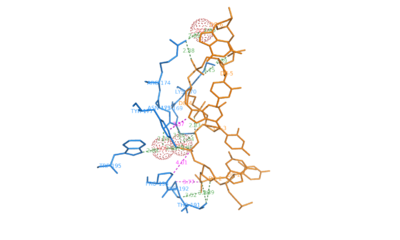
Polar Interactions between ADAR1 and Z-DNA
The double stranded RNA substrate for ADAR1 is formed by folding of 3' intron back onto the exon containing the site to be edited. This shows that the editing of RNA occurs before the splicing of RNA providing an explanation for the binding of Z-DNA by ADAR1. Z-DNA may localize the editing activity of ADAR1 to a particular region within a gene, thus preventing indiscriminate modification. This allows for editing of the nascent transcript and blocks further transcription of gene. It has also been suggested that the extent of adenosine to inosine is proportional to amount of Z-DNA and also the ease with which the surrounding sequences adopt Z-DNA conformation. According to a study binding of ADAR1 to Z-DNA resulted in the increase in promoter activity of the gene which suggests that Z-DNA formation in the promoter region is itself involved in the regulation of transcription.[1]
Vaccinia virus E3L protein
E3L protein of vaccinia virus acts as an immune modulator and is required for replication of the virus. The region of E3L is similar to the Z-alpha domain of ADAR1 but has a lower binding affinity to Z-DNA than ADAR1 or DLM-1. Though the C-terminal of E3L is sufficient for viral replication it is the N-terminal which is responsible for pathogenicity. Mutations or deletions in the N-terminal region reduces the pathogenicity of the virus. Replacement of this domain with its corresponding analogues from ADAR1 or DLM-1 generates a chimeric virus which is as lethal as the wild type virus. Thus a drug which can block the binding of E3L to Z-DNA may be an effective therapy in preventing pathogenicity. Similarity of E3L to variola also suggests that such drugs might be effective against small pox. [2][5] `
DLM-1
DLM-1 is also known as Z-DNA binding protein 1 (ZBP1). It is expressed by a tumor associated gene in lymphatic tissues of mice with mouse ovarian ascites tumor.[2] DLM-1 has two Z-DNA binding domains analogous to the Z-alpha and Z- beta domains in ADAR1. Comparison of Z-DNA binding of DLM-1 and ADAR1 revealed a common structure recognition core within the binding domain. However the role of DLM-1 binding to Z-DNA in tumor development is not known.
Z-DNA binding proteins have common structural characteristics. The binding domains of these proteins can substitute one another and thus can act as competitive inhibitors against one another. As explained above, disruption in the Z-DNA binding region of E3L reduces its pathogenicity. All these observations are important pointers towards the biological importance of Z-DNA.[2]
High-resolution crystal structure of Z-DNA in complex with Cr3+ cations [6]
Trivalent chromium, a d3 cation, is poorly taken up by living cells. The Cr3+ ions are the final product of in vivo Cr6+ metabolism. However, Cr3+ in contrast to Cr6+ can form coordination complexes with macromolecules in the cells. In vitro biochemical experiments have shown that exposure of cells to Cr6+ yields binary (DNA–Cr3+) and ternary (DNA–Cr3+–ligand) adducts, DNA crosslinks, as well as oxidative DNA lesions. Despite the interest in DNA–Cr3+ interactions in biological systems, the existing literature provides detailed crystallographic structural data for only two, low-resolution DNA–Cr3+:DNA polymerase-β complexes, PDB 1zqe (3.7 Å) ֵand 1huz (2.6 ֵÅ).
Our work is part of our project aimed at characterizing metal-binding properties of left-handed Z-DNA helices. The three Cr3+ cations found in the asymmetric unit of the d(CGCGCG)2–Cr3+ crystal structure do not form direct coordination bonds with either the guanine N/O atoms or the phosphate groups of the Z-DNA. (I, green; II, orange) along the DNA chains. . Cr3+ cations shown as purple spheres. Instead, only water-mediated contacts between the nucleic acid and the Cr3+ cations are observed. The coordination spheres of Cr3+(1) and Cr3+(2) contain six water molecules each. The Cr3+(1) and Cr3+(2) ions are bridged by three water molecules from their coordination spheres, one of which (Wat1) is split into two sites. The hydration patterns of Cr3+(1) and Cr3+(2) are (water molecules are represented by red spheres). The Cr3+(3) cation has .
We have used Z-DNA crystals to obtain accurate information about the geometrical parameters characterizing the coordination of Cr3+ ions by left-handed Z-DNA. The d(CGCGCG)2–Cr3+ structure is an excellent illustration of the flexibility of the Z-DNA molecule, visible in the adoption of multiple conformations (by the phosphate groups and the G2 nucleotide), in response to changes in its electrostatic and hydration environment, caused by the introduction of hydrated metal complexes.