We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Chimera of alcohol dehydrogenase by exchange of the cofactor binding domain res 153-295 of C. beijerinckii ADH by T. brockii ADH
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load="3fpl" size="400" color="" frame="true" spin="on" Scene='3fpc/Cv/1' align="right" caption= > 200px {{Template:ABSTRACT_PUBMED_20102159}}...) |
|||
| Line 3: | Line 3: | ||
[[Image:3fpl.jpg|left|200px]] | [[Image:3fpl.jpg|left|200px]] | ||
| - | + | =Chimera Χ22<sub>(CTC)</sub> ([[3fpl]])= | |
{{Template:ABSTRACT_PUBMED_20102159}} | {{Template:ABSTRACT_PUBMED_20102159}} | ||
{{Clear}} | {{Clear}} | ||
| - | |||
| - | |||
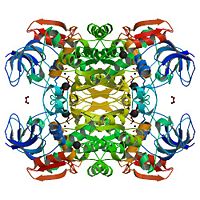
The NADP<sup>+</sup>-dependent [http://en.wikipedia.org/wiki/Alcohol_dehydrogenase alcohol dehydrogenases] ([http://www.expasy.org/enzyme/1.1.1.2 EC 1.1.1.2]) from the [http://en.wikipedia.org/wiki/Thermophile thermophile] ''Thermoanaerobacter brockii'' (TbADH), the [http://en.wikipedia.org/wiki/Mesophile mesophilic] [http://en.wikipedia.org/wiki/Bacteria bacterium] [http://en.wikipedia.org/wiki/Clostridium_beijerinckii ''Clostridium beijerinckii''] (CbADH), and the [http://en.wikipedia.org/wiki/Protozoa protozoan] [http://en.wikipedia.org/wiki/Parasitism parasite] [http://en.wikipedia.org/wiki/Entamoeba_histolytica ''Entamoeba histolytica''] (EhADH1) are <scene name='3fsr/Cv/3'>homotetrameric</scene> [http://en.wikipedia.org/wiki/Tetrameric_protein] ([http://en.wikipedia.org/wiki/Protein_subunit monomers] are colored in different colors) secondary alcohol dehydrogenases. Each <scene name='3fsr/Cv/4'>monomer</scene> of these alcohol dehydrogenases consists of two domains: the <scene name='3fsr/Cv/5'>cofactor-binding domain</scene> <font color='blueviolet'><b> (residues 154−294 for TbADH)</b></font> and the <scene name='3fsr/Cv/6'>catalytic domain</scene> (<font color='red'><b>residues 1−153 and 295−351 for TbADH</b></font>; contains [http://en.wikipedia.org/wiki/Zinc Zn<sup>2+</sup>] at the [http://en.wikipedia.org/wiki/Active_site active site]) separated by a deep cleft. Although, all these three ADHs revealed a high degree of [http://en.wikipedia.org/wiki/Conserved_sequence sequence conservation] (62-75% identity), them significantly differ in [http://en.wikipedia.org/wiki/Thermostability thermostability]. The [http://en.wikipedia.org/wiki/Cofactor_(biochemistry) cofactor]-binding domains (residues 153−295) of TbADH, CbADH, and EhADH1 were mutually <scene name='3fsr/Cv/7'>exchanged</scene> and 3 corresponding chimeras were constructed. | The NADP<sup>+</sup>-dependent [http://en.wikipedia.org/wiki/Alcohol_dehydrogenase alcohol dehydrogenases] ([http://www.expasy.org/enzyme/1.1.1.2 EC 1.1.1.2]) from the [http://en.wikipedia.org/wiki/Thermophile thermophile] ''Thermoanaerobacter brockii'' (TbADH), the [http://en.wikipedia.org/wiki/Mesophile mesophilic] [http://en.wikipedia.org/wiki/Bacteria bacterium] [http://en.wikipedia.org/wiki/Clostridium_beijerinckii ''Clostridium beijerinckii''] (CbADH), and the [http://en.wikipedia.org/wiki/Protozoa protozoan] [http://en.wikipedia.org/wiki/Parasitism parasite] [http://en.wikipedia.org/wiki/Entamoeba_histolytica ''Entamoeba histolytica''] (EhADH1) are <scene name='3fsr/Cv/3'>homotetrameric</scene> [http://en.wikipedia.org/wiki/Tetrameric_protein] ([http://en.wikipedia.org/wiki/Protein_subunit monomers] are colored in different colors) secondary alcohol dehydrogenases. Each <scene name='3fsr/Cv/4'>monomer</scene> of these alcohol dehydrogenases consists of two domains: the <scene name='3fsr/Cv/5'>cofactor-binding domain</scene> <font color='blueviolet'><b> (residues 154−294 for TbADH)</b></font> and the <scene name='3fsr/Cv/6'>catalytic domain</scene> (<font color='red'><b>residues 1−153 and 295−351 for TbADH</b></font>; contains [http://en.wikipedia.org/wiki/Zinc Zn<sup>2+</sup>] at the [http://en.wikipedia.org/wiki/Active_site active site]) separated by a deep cleft. Although, all these three ADHs revealed a high degree of [http://en.wikipedia.org/wiki/Conserved_sequence sequence conservation] (62-75% identity), them significantly differ in [http://en.wikipedia.org/wiki/Thermostability thermostability]. The [http://en.wikipedia.org/wiki/Cofactor_(biochemistry) cofactor]-binding domains (residues 153−295) of TbADH, CbADH, and EhADH1 were mutually <scene name='3fsr/Cv/7'>exchanged</scene> and 3 corresponding chimeras were constructed. | ||
| - | The cofactor-binding domain of thermophilic TbADH was replaced with the cofactor-binding domain of its mesophilic counterpart CbADH (chimera Χ21<sub>(TCT)</sub>, [[3fsr]]). This domain replacement significantly destabilized the parent thermophilic enzyme (ΔT<sub>1/2</sub> = −18 °C). But the reverse exchange in CbADH (chimera Χ22<sub>(CTC)</sub>, [[3fpl]]), had little effect on the thermal stability of the parent mesophilic protein | + | The cofactor-binding domain of thermophilic TbADH was replaced with the cofactor-binding domain of its mesophilic counterpart CbADH (chimera Χ21<sub>(TCT)</sub>, [[3fsr]]). This domain replacement significantly destabilized the parent thermophilic enzyme (ΔT<sub>1/2</sub> = −18 °C). But the reverse exchange in CbADH (chimera Χ22<sub>(CTC)</sub>, [[3fpl]]), had little effect on the thermal stability of the parent mesophilic protein. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
{{Clear}} | {{Clear}} | ||
Revision as of 10:54, 12 June 2012
| |||||||||||
Reference
Biochemical and structural properties of chimeras constructed by exchange of cofactor binding domains in alcohol dehydrogenases from thermophilic and mesophilic microorganisms., Goihberg E, Peretz M, Tel-Or S, Dym O, Shimon LJ, Frolow F, Burstein Y.Biochemistry. 2010 Jan 26. Epub ahead of print
- (See also Tetrameric alcohol dehydrogenases)
Categories: Thermoanaerobacter brockii, entamoeba histolytica | Burstein, Y. | Felix, F. | Goihberg, E. | Shimon, L. | Bacterial alcohol dehydrogenase | Chimera | Cytoplasm | Domain exchange | Metal-binding | Nadp | Oxidoreductase | Zinc | ISPC, Israel Structural Proteomics Center. | Peretz, M. | Tel-Or, S. | ISPC | Israel Structural Proteomics Center | Structural genomic