DAHP synthase
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== Function == | == Function == | ||
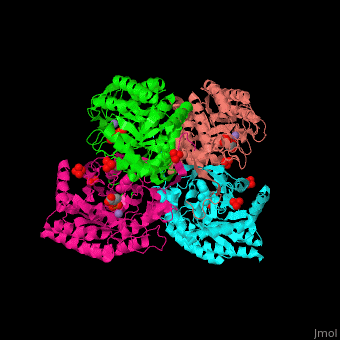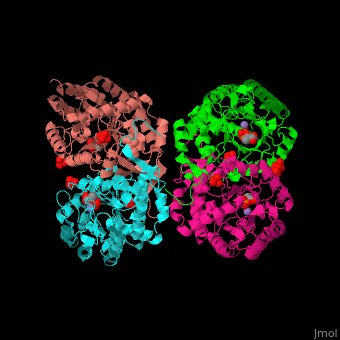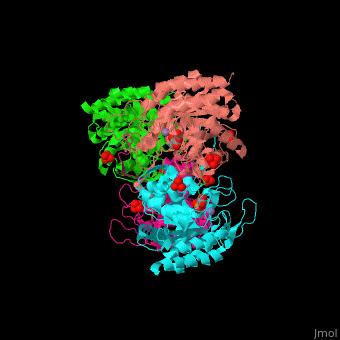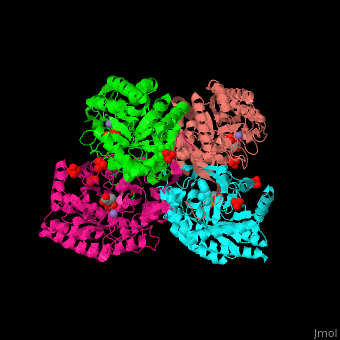
| - | '''DAHP synthase''' or '''3-deoxy-D-arabino-heptulosonate 7-phosphate synthase''' (DAHPS) catalyzes the conversion of phosphoenolpyruvate (PEP) and D-erythrose 4-phosphate to 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) and phosphate. DAHPS is part of the shikimate pathway. DAHPS requires a bivalent metal ion cofactor for normal activity. <scene name='70/708806/Cv/ | + | '''DAHP synthase''' or '''3-deoxy-D-arabino-heptulosonate 7-phosphate synthase''' (DAHPS) catalyzes the conversion of phosphoenolpyruvate (PEP) and D-erythrose 4-phosphate to 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) and phosphate. DAHPS is part of the shikimate pathway. DAHPS requires a bivalent metal ion cofactor for normal activity. <scene name='70/708806/Cv/6'>DAHPS is a tetramer</scene>. DAHPS exhibits feedback inhibition by aromatic amino acids like tyrosine, phenylalanine and tryptophan.<ref>PMID:1682314</ref> |
== Structural highlights == | == Structural highlights == | ||
| - | The DAHPS active site is located in a channel at the C-terminal of the enzyme where the <scene name='70/708806/Cv/ | + | The DAHPS active site is located in a channel at the C-terminal of the enzyme where the <scene name='70/708806/Cv/7'>substrate (PEP)</scene>, <scene name='70/708806/Cv/8'>inhibitor (phenylalanine)</scene> and <scene name='70/708806/Cv/9'>metal ion (Mn+2)</scene> are seen. The bivalent metal is bound to a Cys-X-X-His motif.<ref>PMID:12126632</ref> |
</StructureSection> | </StructureSection> | ||
Revision as of 10:39, 21 February 2019
| |||||||||||
3D Structures of DAHP synthase
Updated on 21-February-2019
References
- ↑ Stephens CM, Bauerle R. Analysis of the metal requirement of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase from Escherichia coli. J Biol Chem. 1991 Nov 5;266(31):20810-7. PMID:1682314
- ↑ Shumilin IA, Zhao C, Bauerle R, Kretsinger RH. Allosteric inhibition of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase alters the coordination of both substrates. J Mol Biol. 2002 Jul 26;320(5):1147-56. PMID:12126632