Structural highlights
Function
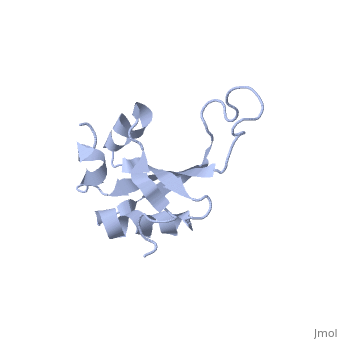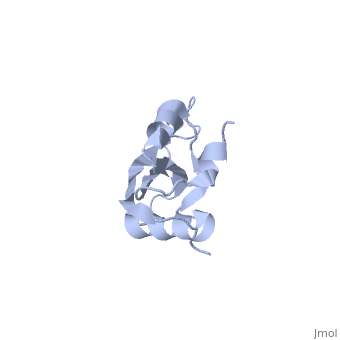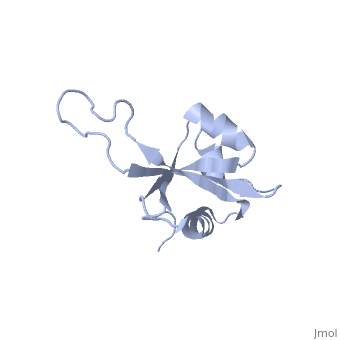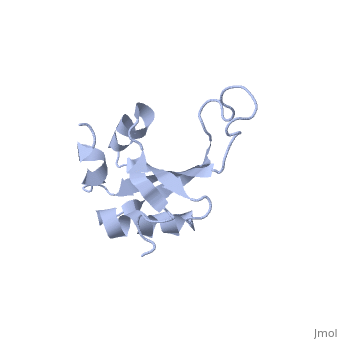
RL23_THETH One of the early assembly proteins it binds 23S rRNA. One of the proteins that surrounds the polypeptide exit tunnel on the outside of the ribosome. Forms the main docking site for trigger factor binding to the ribosome (By similarity).
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The ribosomal protein L23 is a component of the large ribosomal subunit in which it is located close to the peptide exit tunnel. In this position L23 plays a central role both for protein secretion and folding. We have determined the solution structure of L23 from Thermus thermophilus. Uncomplexed L23 consists of a well-ordered part, with four anti-parallel beta-strands and three alpha-helices connected as beta-alpha-beta-alpha-beta-beta-alpha, and a large and flexible loop inserted between the third and fourth beta-strand. The observed topology is distantly related to previously known structures, primarily within the area of RNA biochemistry. A comparison with RNA-complexed crystal structures of L23 from T. thermophilus, Deinococcus radiodurans and Haloarcula marismourtui, shows that the conformation of the well-ordered part is very similar in the uncomplexed and complexed states. However, the flexible loop found in the uncomplexed solution structure forms a rigid extended structure in the complexed crystal structures as it interacts with rRNA and becomes part of the exit tunnel wall. Structural characteristics of importance for the interaction with rRNA and with the ribosomal protein L29, as well as the functional role of L23, are discussed.
NMR structure of the ribosomal protein L23 from Thermus thermophilus.,Ohman A, Rak A, Dontsova M, Garber MB, Hard T J Biomol NMR. 2003 Jun;26(2):131-7. PMID:12766408[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Ohman A, Rak A, Dontsova M, Garber MB, Hard T. NMR structure of the ribosomal protein L23 from Thermus thermophilus. J Biomol NMR. 2003 Jun;26(2):131-7. PMID:12766408