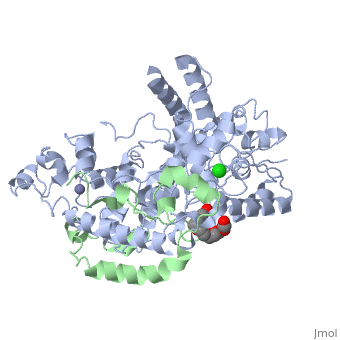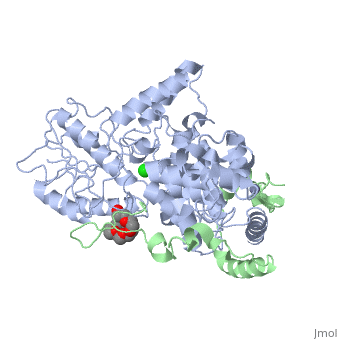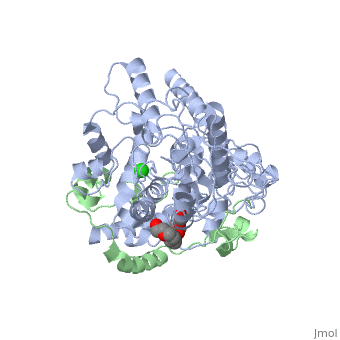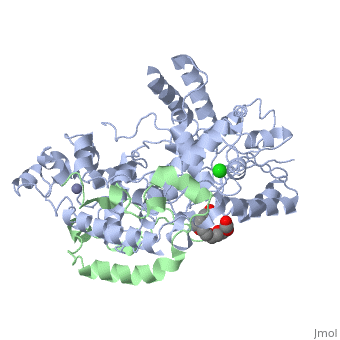
| Structural highlights
Function
CRY1_MOUSE Blue light-dependent regulator of the circadian feedback loop. Inhibits CLOCK|NPAS2-ARNTL E box-mediated transcription. Acts, in conjunction with CRY2, in maintaining period length and circadian rhythmicity. Has no photolyase activity. Capable of translocating circadian clock core proteins such as PER proteins to the nucleus. May inhibit CLOCK|NPAS2-ARNTL transcriptional activity through stabilizing the unphosphorylated form of ARNTL.[1] [2] [3]
Publication Abstract from PubMed
Period (PER) proteins are essential components of the mammalian circadian clock. They form complexes with cryptochromes (CRY), which negatively regulate CLOCK/BMAL1-dependent transactivation of clock and clock-controlled genes. To define the roles of mammalian CRY/PER complexes in the circadian clock, we have determined the crystal structure of a complex comprising the photolyase homology region of mouse CRY1 (mCRY1) and a C-terminal mouse PER2 (mPER2) fragment. mPER2 winds around the helical mCRY1 domain covering the binding sites of FBXL3 and CLOCK/BMAL1, but not the FAD binding pocket. Our structure revealed an unexpected zinc ion in one interface, which stabilizes mCRY1-mPER2 interactions in vivo. We provide evidence that mCRY1/mPER2 complex formation is modulated by an interplay of zinc binding and mCRY1 disulfide bond formation, which may be influenced by the redox state of the cell. Our studies may allow for the development of circadian and metabolic modulators.
Interaction of Circadian Clock Proteins CRY1 and PER2 Is Modulated by Zinc Binding and Disulfide Bond Formation.,Schmalen I, Reischl S, Wallach T, Klemz R, Grudziecki A, Prabu JR, Benda C, Kramer A, Wolf E Cell. 2014 May 22;157(5):1203-15. doi: 10.1016/j.cell.2014.03.057. PMID:24855952[4]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999 Jul 23;98(2):193-205. PMID:10428031
- ↑ Kondratov RV, Kondratova AA, Lee C, Gorbacheva VY, Chernov MV, Antoch MP. Post-translational regulation of circadian transcriptional CLOCK(NPAS2)/BMAL1 complex by CRYPTOCHROMES. Cell Cycle. 2006 Apr;5(8):890-5. Epub 2006 Apr 17. PMID:16628007
- ↑ Chaves I, Yagita K, Barnhoorn S, Okamura H, van der Horst GT, Tamanini F. Functional evolution of the photolyase/cryptochrome protein family: importance of the C terminus of mammalian CRY1 for circadian core oscillator performance. Mol Cell Biol. 2006 Mar;26(5):1743-53. PMID:16478995 doi:10.1128/MCB.26.5.1743-1753.2006
- ↑ Schmalen I, Reischl S, Wallach T, Klemz R, Grudziecki A, Prabu JR, Benda C, Kramer A, Wolf E. Interaction of Circadian Clock Proteins CRY1 and PER2 Is Modulated by Zinc Binding and Disulfide Bond Formation. Cell. 2014 May 22;157(5):1203-15. doi: 10.1016/j.cell.2014.03.057. PMID:24855952 doi:http://dx.doi.org/10.1016/j.cell.2014.03.057
|