Introduction
Anthrax Lethal Factor (LF) is one of the enzymatic components belonging to the Anthrax Toxin. Anthrax toxin is a three component exotoxin secreted by the bacterium Bacillus Anthracis made up of a binding protein, protective antigen (PA) and two enzyme components edema factor (EF) and lethal factor (LF). [1] See also Toxins.
Anthrax Toxin, encoded by plasmid pXO1, is considered an AB toxin, with two A domains (EF and LF) and one B domain (PA). [1] [2] On their own, these three domains are nontoxic, but any combination involving PA with EF and/or LF is what causes the physiological effects. [3] Initially PA is a 83kDa protein which binds to the host Anthrax toxin Receptor (ATR). Upon binding, PA is cleaved into two fragments by furin proteases to become a 63 kDa protein bound to the ATR. Cleavage of PA allows self association to occur which forms a ring shaped heptamer know as the pore precursor (prepore). The prepore is now able to bind up to three molecules of EF and/or LF, leading to endocytosis of the complex. In the endosome, the prepore converts to a transmembrane pore, allowing translocation of EF and LF to the cytosol of target cell through a mechanism that is not well understood. EF and LF are now able to carry out their enzymatic activity on the host cell. [1]
EF is a calmodulin and calcium dependent adenylate cyclase that increases cAMP to extraordinary levels in cells. Changes in intracellular cAMP affect membrane permeability and may account for edema. In macrophages and neutrophils an additional effect is the depletion of ATP reserves which are needed for the engulfment process. [1] [4] [5]
LF is a Zinc dependent protease that cleaves certain MAP kinase kinases (MAPKK)leading to the disruption of many cellular signalling pathways, which eventually leads to cell death. [1] [6]
This is a list of the possible combinations that can occur with Anthrax Toxin: [7]
PA+LF Leads to lethal activity
EF+PA Leads to edema
EF+LF Non-toxic
PA+LF+EF Leads to lethal activity and edema
See also Anthrax Lethal Factor (hebrew).
Human Interaction
Anthrax is primarily a disease of domesticated and wild animals. Herbivores such as cattle, sheep, horses, mules and goats are primarily affected because these animals may be grazing on soils contaminated with Bacillus Anthracis endospores. [1] [8] The blood of an animal that dies of anthrax can contain upward of 10^9 vegetative bacteria per milliliter and as the carcass decays, the bacteria form highly infectious endospores, which contaminate the local environment and can remain viable for long time periods. [1] The endosopres produced by Bacillus Anthracis remains viable for lengthy periods due to the poly-D-glutamic acid capsule, which itself is nontoxic. This capsule functions to protect the endospore from complement and other bactericidal components found in serum. This capsule plays an important role during the infection of anthrax, but is not important during the disease phase, which is caused by PA, EF, LF. Genes encoding the capsule are located on plasmid pXO1. [1] [9] Anthrax is not a common disease among humans because it can not be transmittable from human to human. Humans become infected by being exposed to farm animals or contaminated animal products such as wool, hides, flesh and blood. There are three ways in which Anthrax can be transmitted to humans: [1] [10]
Cutaneous Anthrax is the most common form of the disease. This usually occurs when endospores enter the body through injured skin and germinates. In most cases the bacteria remain contain at the site of infection and present as a lesion. In rare case, the infection could spread to the blood stream and cause septicemia. Treatment is often not needed. [11]
Gastrointestinal Anthrax occurs through the ingestion of spore contaminated meat. The spores then invade the mucosa through a preexisting lesion. After germination, spores spread from the mucosal lesion into the lymphatic system. This form of Anthrax is associated with a high mortality rate but is considered the rarest of the three types of infection. [12]
Inhalation Anthrax is the most fatal of the three infections. Also know as woolsorters' disease, this form involves the inhalation of spore usually contained in animal hair and hides. The spores colonize the alveolar macrophages and its believe the macrophages serve both as the sites of germination and as vehicles for transporting the bacteria. At this point the bacteria can rapidly spread throughout the body. If left untreated death is certain. Even with antibiotics mortality rates are high. [13]
Treatments
Antibiotics are used to treat Cutaneous, if infection spreads and Inhalation Anthrax infections. The primary antibiotics used are Ciprofloxacin and Doxycycline. Antibiotics should be administered before symptoms arise, because it will decrease the fatality rate. In the case of inhalation, treatment should be received within 24 hours because the bacteria can rapidly spread. Treatment includes a 60 day course of antibiotics to ensure all spores have germinated. In some cases more than two antibiotics are administered. [14].
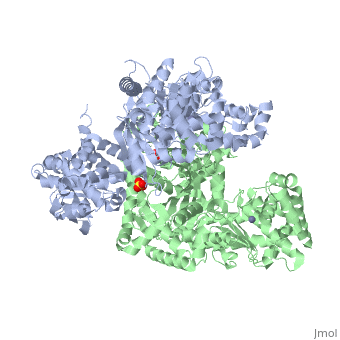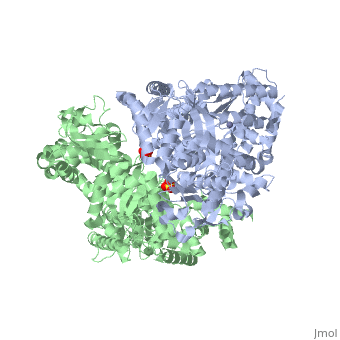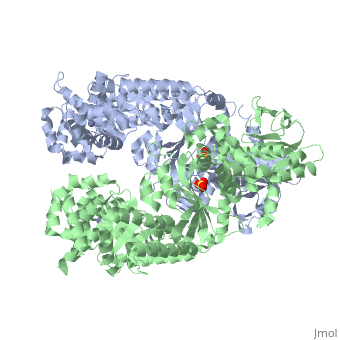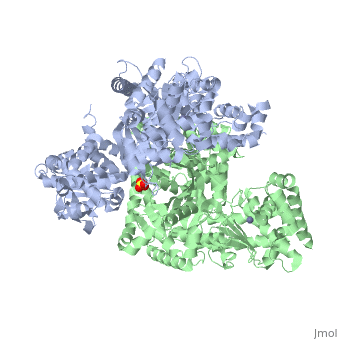
Structure of Lethal Factor
Anthrax Lethal Factor is composed of four domains:
binds Lethal Factor to Protective Antigen 63 (PA63), which is the membrane translocation component of Anthrax Toxin, but the actual location where domain I interacts with PA is unknown. [1] Domain I(residues 1-263) is perched above the other three domains and is connected to the rest of the domains through an abrupt turn at the end of the last helix.[1] Domain I consist of 12-helix bundle, packs against one face of a mixed four-stranded beta-sheet.[1] [15]
(residues 263-297 and 385-550), has a similar structure with that of Bacillus Cereus toxin catalytic domain VIP2, which contains a NAD binding pocket, but domain II lacks ADP-ribosylating activity due to the lack of conserved residues. There is approximately 15% sequence similarity between them. [1] [16]
(residues 303-383), contains a segment of five tandem repeats residues 282-382. This suggests that repeats 2-5 arose from a duplication of repeat 1, which is the second helix-turn element of domain II. Repeats 2-5 form the four helix-turn elements of the helical bundle. This domain is required LF activity, shares the same hydrophobic surface as domain IV and its location restricts access to the active site. Also, it contributes to substrate specificity by making interactions with the substrate. [1][17]
(residues 552-776), consists of a nine-helix bundles packed against a four stranded beta-sheet, containing a HExxH motif. The first six helices and the beta-sheet can be superposed with those of the metalloprotease. A zinc ion is coordinated tetrahedrally by a water molecule and three protein side chains in an arrangement typical of the thermolysin family. Two of the coordinating residues are the histidines from the HExxH motif (His 686 and His 690) and Glu 735. [1] [18]
Domains II, III, and IV for the for the substrate.
Function of Lethal Factor
The MAPKK family of proteins are the only known cellular substrates of LF. LF cleaves near their N termini removing the docking sequence for the downstream MAP kinase. At low levels of LF, MAPKK-3 is cleaved inhibiting release of pro-inflammatory mediators. In contrast, high levels of LF lead to lysis of macrophages within a few hours, by an unknown mechanism. This suggests during early infection there is a delayed immune response while in the late stage of infection bacterium in the bloodstream trigger macrophage lysis and the sudden release of high levels pro-inflammatory mediators. This is consistent with the septic shock symptoms seen before death. [1] [19]
A zinc ion (Zn2+) is coordinated tetrahedrally by a water molecule and three protein side chains, His 686, His 690 and Glu 735. Glu 687 from the HExxH motif lies 3.5A from the water molecule, making it well positioned to act as a general base to activate the zinc-bound water during catalysis. The hydroxyl group of a tyrosine residue (Tyr 728) forms a strong hydrogen bond to the water molecule, on the opposite side of Glu 687, and probably functions as a general acid to protonate the amine leaving group. The binding pocket is not closed at its N-terminal end, so longer tails other MAPKK simply protrude beyond the pocket.[1] [20] [21]
Biowarfare
The most will known case of biological warfare in recent times occurred in 2001, known as Amerithrax. Anthrax spores are a top choice for biological warfare because their potency. Production of these spores are among the easiest of bioterror agents. [22] They can be manipulated and produced in large quantities using basic microbiology techniques. Naturally occurring spores tend to aggregate making them less infection; they can easily be refined and dispersed. Because the anthrax spores are very robust; being able to survive for decades and are difficult to destroy, makes them excellent bioweapons. [23] [24]
3D structures of anthrax lethal factor
Anthrax lethal factor 3D structures