Delta-endotoxin named also Cyt or Cry, is a pore-forming toxin produced by Bacillus thuringiensis. It is used as an insecticide. Upon ingestion by insect, δ-endotoxin is cleaved, binds to the gut epithelium and forms cation channels. This causes cell lysis and death. For a list of various toxins see Toxins.
See also Cyt1Aa Toxin: High Resolution Structure Reveals Implications for its Membrane-Perforating Function
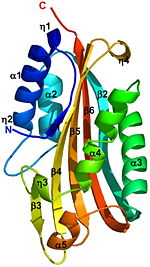
δ-endotoxin from Bacillus thuringiensis
The crystal structure of the proteolytically activated monomeric form of Cyt2Ba was determined at 1.8Å resolution. It consists of a single domain of architecture with a (yellow) surrounded by 2 layers (red) forming a cytolysin fold. The β-sheet is comprised of 6 anti-parallel β-strands (β1-β6). On one side of this sheet there is an α-helix layer consisting of α1, α2; and on the other side a second α-helix layer, composed of α3-α5. The β-strands β2-β5 of the central β-sheet have a modified Greek-key topology. The Greek key motif consists of four adjacent antiparallel strands and their linking loops. It consists of three antiparallel strands connected by hairpins, while the fourth is adjacent to the first and linked to the third by a longer loop [1]. Cyt2Ba (gray) has only 16% sequence identity with VVA2 (colored red, 1pp0), however they both have a cytolysin fold and their overall structure is very similar (see their ).
A remarkable similarity is observed between the structures of the endogenously cleaved Cyt2Ba (gray) and the (red) within the inactive protoxin of Cyt2Aa (1cby, monomers A and B of Cyt2Aa shown red and blue, respectively, the N- and C-termini are shown in spacefilling representation). Although, 1cby is a 1 chain structure, the biological relevant molecule for 1cby can be assembled from the contents of the deposited coordinates by the application of crystallographic symmetry operations to give a dimer. It can be downloaded. Each monomer of Cyt2Aa (1cby), consists of an additional β-strand at its N-terminus and an additional α-helix at its C-terminus compared to the cleaved Cyt2Ba. The of Cyt2Aa is held together by the intertwined N-terminal strands from both monomers. The cleavage of Cyt2Aa the N- and C-terminal segments, prevents dimer formation and releases an . Similarly, in Cyt2Ba the proteolysis causes the removal of 34 amino acids at its N-terminus and 28 or 30 residues at its C-terminus forming the crystallized toxic monomer.
The crystal structure of monomeric Cyt2Ba is the first structure of a toxic form of the Cyt family. Its structure is homologous to the corresponding region of Cyt2Aa and to that of VVA2. This structural comparison shows that the toxicity of Cyt2Ba, Cyt2Aa and VVA2 is an inherent property of the monomer and not the result of secondary structure rearrangement upon cleavage. Solving the 3D structure of these proteins extends the knowledge of the cytolytic machinery of pore-forming toxins and helps in designing novel membrane-active cytotoxins.
The toxicity to insects of the gram-positive bacterium Bacillus thuringiensis, widely used as a biological alternative to chemical pesticides, is due to δ-endotoxic crystals comprised of a series of proteins that react with the cells lining the larval midgut of susceptible insects. The insecticidal proteins are produced during sporulation and classified into two families of membrane perforating toxins, Crystal (Cry) and Cytolytic (Cyt), that are packed into a para-crystalline structure. Following ingestion by an insect of its host range, the Cry and Cyt toxic crystals are solubilized and their pro-toxins are cleaved by the alkaline-active digestive enzymes at the high pH prevailing in the larval midgut. The activated Cry toxins bind to specific protein receptors located on the host cell surface, oligomerize and insert into the membrane, forming lytic pores that cause cell swelling and lysis. In contrast, Cyt toxins do not bind specific receptors but act non-specifically by direct interaction with membrane lipids. However, there is assumption that the toxicity of Cyt1A may be related to the specific unsaturated fatty acid composition of lipids in the midgut epithelial cells of dipteran insects. The two Cyt and Cry families share no common sequence or structural resemblance. They have distinct secondary structures: the α-helical regions of the Cry toxins form the trans-membrane pore, whereas Cyt toxins are presumed to be inserted into the membrane by a β-barrel composed of β-sheet hairpins from each monomer. The activated monomeric form of Cyt1Aa, the most toxic Cyt family member, was isolated and crystallized, and its structure was determined at 2.2 Å resolution (PDB code 3ron). Cyt1Aa adopts a containing a (yellow) held by (red).
The conventional model for the Cyt proteins suggests that the monomer undergoes conformational changes, such that (sites of swing labeled in blue). Oligomerization of Cyt monomers on the cell membrane forming β-barrel pores.
Cyt1Aa (lime), like other Cyt family members, also has a (cyan, PDB code 1vcy) and the non-toxic virulence factor Evf (PDB code 2w3y) despite their very low sequence identity. While, Evf is covalently bound to palmitate, none of the Cyt family members contain a palmitoylated Cys residue. The structural homology between Cyt1Aa and Evf enabled the identification of a . The Cyt1Aa structure displays the (colored in magenta) pointing towards the putative lipid-binding pocket. We suggest that in Evf, the covalently bound lipid “locks” the helical layer to the β-sheet and prevents the conformational changes necessary for membrane insertion, explaining its observed non-toxicity. On the other hand, the absence of the lipid in Cyt1Aa enables its flexibility and allows the conformational changes of the two surrounding α-helical layers of Cyt1Aa necessary for exposing the hydrophobic β-sheet which is necessary prior to their membrane insertion and perforation.
In attempt to understand why Cyt1Ca is non-toxic, we performed a comparative sequence analysis of all known Cyt1 family members revealing that Cyt1Ca is the most divergent. The residues that are conserved in Cyt1Aa, Cyt1Ab and Cyt1Ba but differ in Cyt1Ca are located on the α-helical layers and on strands β1, β4 and β5 which have been proposed to undergo conformational changes upon membrane binding. The contribution of these residues to the lack of toxicity of Cyt1Ca was supported by the finding that mutating three of these non-conserved residues, Q154, Q164, and G240 in Cyt1Ca to the corresponding charged and exposed residues in Cyt1Aa, K154, E164, and D240 respectively, restored partial antibacterial though not larvicidal activities indicating their importance. We suggest that the lack of its toxicity may also be related to its lack of flexibility. This is supported by the finding that substitution of Q225 in Cyt1Ca to the corresponding conserved K225 in Cyt1Aa, does not restore activity. This residue is located on β8, which is part of the sheet thought to insert into the membrane. We postulate that the location of the non-conserved residues in Cyt1Ca may render this protein unable to undergo the conformational changes associated with membrane insertion, thereby explaining its non-toxicity.
Cyt1Aa synergizes activities of Cry11Aa. Two binding epitopes of Cyt1Aa, (locating on β7 and α6) and (locating on β8), were found to be involved in the binding interaction with Cry11Aa. Both regions are mostly embedded, with only exposed (colored in blueviolet). The role of these epitopes was confirmed by heterologous competition assays using synthetic peptides. corresponding to these regions and by site directed mutagenesis. In particular, . Recently it has been shown that mutation of these Cyt1Aa residues affect its binding and synergism with Cry4Ba as well. Interestingly, these three residues are charged in most of the Cyt1 family members, whereas in the Cyt2 family and in Cyt1Ca, which presumably do not bind Cry11Aa, they are polar (T198, Q204 and T225 respectively in Cyt2Ba). Thus, it seems reasonable that synergism and binding of Cyt1Aa to Cry11Aa or to Cry4Ba depend on specific interactions between these toxins, which involve these residues. We suggest that the reduced charge on the Cyt2 protein members and on Cyt1Ca may be sufficient to abrogate binding to Cry11Aa. It was suggested that mutating these residues in other Cyt proteins to the corresponding Cyt1Aa charged residues might introduce binding sites and induce synergism with Cry toxins. This strategy could be used as a tool to overcome Cry-resistance in the midgut membrane of resistant insects. A sequential mechanism has been proposed by which Cyt1Aa initially undergoes conformational changes to insert its β-sheet into the membrane following binding of Cry11Aa via the two Cyt1Aa binding epitopes resulting in insertion of Cry11Aa into the mosquito membranes. Mapping the three charged residues on the Cyt1Aa structure revealed that while all three residues are exposed to the surface of the protein, they all reside on regions of the toxin which presumably are inserted into the membrane (K198 and E204 are located on β7 and α6, and K225 is part of β8). We therefore, can't out rule an alternative mechanism by which Cyt1Aa binds Cry11Aa using these exposed charged residues prior to its membrane insertion. Thus, the action of Cyt1Aa alone or as a receptor for Cry11Aa may involve different mechanisms.
The pattern of the hemolytic activity of Cyt1Aa presented here (resembling that of pore-forming agents), while differing from that imposed by ionic and nonionic detergents, further supports the pore-forming model by which conformational changes occur prior to membrane insertion and perforation.