FOXP3 mutation- IPEX syndrome
From Proteopedia
FOXP3 mutation- IPEX syndrome
Immune dysregulation, polyendocrinopathy, entheropathy, X-linked syndrome is rare autoimmune disorder caused by genetic mutation in FOXP3 Forkhead box protein gene, which is responsible for producing important transcription factor required for maintenance of T regulatory cells (T-regs). T-reg cells disfunction is main pathogenic event which leads to multiorgan autoimmunity called IPEX syndrome. [1]
FOXP3 gene structure
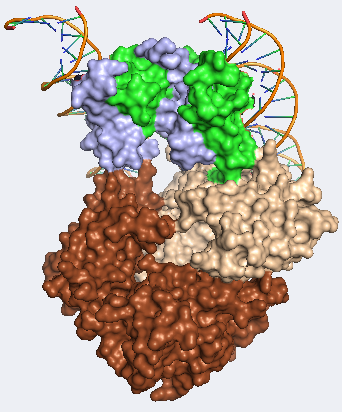
FOXP3 gene is located on centromeric region of the X chromosome in position Xq11.3-q13.3 and is comprised of 12 exones. Molecular location is from 49,250,436- 49,264,932 base pairs on the X chromosome. This gene can be also found under other names such as: AIID, DIETER, IPEX, scurfin…[2] FOXP3 is highly conserved gene responsible for production of 431 amino acids long fork head protein P3 (FOXP3 protein). This protein is a member of the FKH family of transcription factors. Contains proline rich (PRR) aminoterminal domain, central zinc finger (ZC), leucine zipper domain and carboxyl terminal FKH domain. [3]
Function
Probably the most characteristic part of FOXP3 protein is the “forkhead box”. This part of the protein, consisting of 80-100 amino acids, form a DNA binding motif, giving FOXP3 protein capacity to bind DNA and regulate expression of various genes and is highly expressed in CD4+CD25+ regulatory T cells (T-regs). FOXP3 is able bind to a number of distinct genomic loci in T-regs and therefore can function as both repressor and trans-activator depending on the interaction partners.[4] This specific T cell subset is involved in limiting the immune response of other cells, for example conventional cytotoxic T cells. T-regs are developed from relatively small population of CD4+T helper cells in thymus. They are characterized by surface markers such as CD25 or CTLA4, but most importantly FOXP3 which is functionally involved in immunosuppression and therefore without FOXP3 protein present T-reg cells do not develop at all.[5]
For more information on structure and function see also #REDIRECT Forkhead Box Protein 3
FOXP3 mutations
Currently we know two options that can lead to IPEX syndrome. Either it happens due to deletion of FOXP3 gene in a germ line, which subsequently leads to complete loss of T regulatory cells or it can be caused by missense mutations that allow for some Treg development although with limited capabilities. Up to this day there are at least 70 distinct FOXP3 mutations known to cause IPEX. Out of all identified mutations 40% reside in C-terminal FKH DNA binding domain, 23% in N-terminal PRR domain, 9% in the LZ domain, 16% in the LZ-FKH loop, 6% in the noncoding region upstream of initiating ATG and last 6% in C- terminal end of the ORF. However, among different patient same mutation can be responsible for dramatically different phenotypes. For example, mutation in FKH domain (c.1150G>A) is connected with patients surviving more than 10 years, but there are also reported cases when patient died prematurely. Severe phenotype was usually observed at individuals with completely impaired expression of functional FOXP3 protein (frameshift, missplicing…). [6] One example of missense mutation is mutation reported in R337Q with deleterious effects on FOXP3 protein functions resulting in pathogenic conditions. In a functional FOXP3 protein arginine 377 is predicted to be responsible for close binding with DNA backbone. Strong basic residues at this place are present and conserved across all Forkhead transcription factors and provides contribution to their DNA binding abilities via both hydrogen bonding and electrostatic interactions. However, substitution in R337Q results in impaired close contact with DNA backbone and presumably causes profound loss of positive charge at the FOXP3 protein DNA binding surface. Other examples of mutation with deleterious effect on DNA binding ability may be P339A. Where proline, original present at 339 position, lies in a Y-turn between A337 and DNA binding domain. It's substitution for alanine therefore most likely influence the topology of A337 and helix 1 in connection with DNA and result in impaired DNA binding and result in severe cases of IPEX. In contrast, replacement of valine with methionine on position 408 results only in mild form of IPEX. This is due to methionine, on his new position at β-turn at the COOH-terminal of wing 1 (where it replaces valine), being capable of creating Van Der Waals contact with opposite chain of Helix 1. This result in reduced flexibility of DNA binding domain of FOXP3 and therefore influence affinity for DNA. However given the fact that nature of this interaction is not able to influence surface charge distribution or jeopardize hydrogen bond with T406 the clinical features of resulting phenotype are mild. Yet another kind of mutation pose deletion in 227 region that leads to frameshift and creation of premature stop codon. Resulting protein is completely inactive due to lack of Forkhead domain, leucine zipper domains and zinc finger. [7]
IPEX phenotype and clinical manifestations
Most cases of IPEX are born at term with normal weight and length. However, first signs of illness may present itself immediately after birth or in the very first days of life. This suggests that autoimmune process has been already initiated in utero. Some case reportedly developed intrauterine growth retardation and shortly after birth they developed also standard IPEX phenotype. Severe symptoms with early onset can be rapidly fatal with absence of early diagnostics and treatment. Most common symptoms are diarrhea, type 1 diabetes and eczema, however overall picture can be complicated with other autoimmune symptoms. Development of Autoimmune enteropathy is one of the key hallmarks of the disease presenting itself as neonatal watery diarrhea with traces of mucus and blood. This symptom starts with breastfeeding and usually worsens when baby starts implementing regular nutrition which may result in severe malabsorption. Type one diabetes can precede or follow enteritis. Similar to diabetes and diarrhea cutaneous manifestations are also very common and may present shortly after birth and can range from mild dermatitis to severe and diffuse lesions. These lesions might be accompanied by bacterial infections. Other symptoms that may be present are thyroid dysfunction, autoimmune cytopenia, autoimmune hemolytic anemia, renal disease, arthritis (involving one or more joints).[8]
Diagnostics
For a correct diagnosis family history is of a great value. However, the symptoms of the disease may vary within family members. This may be caused by different regulatory elements, differences in gene modifications, different environment or previous treatment. Among clinical symptoms signs of type one diabetes, hypothyroidism, enteropathy and cytopenia may be present. Histopathology analysis shows complete absence of normal mucosa in the small bowel and colon sometimes with infiltrations of inflammatory cells in the lamina propria and submucosa as well as in other organs including pancreas, skin or kidneys[9]. From the blood autoantibodies can be detected against pancreas, thyroid and erythrocytes.[10] Changes in complement elements, granulocytes conventional T cells or immunoglobulins have not been reported. This however may not be enough for disease confirmation. For final differential diagnostics has to be based on DNA analysis which confirms mutations in FOXP3 gene.[11]
Treatment
As for now, the most promising treatment comes from combination of immunosuppressive therapy[12] and bone marrow transplantation[13] with addition of supportive treatment including treatment of diabetes and parenteral nutrition. IPEX syndrome is also a good candidate for gene therapy treatment, however, still needs more preclinical studies to evaluate safety and efficiency. [14]
References
- ↑ Bacchetta, R., Barzaghi, F., & Roncarolo, M.-G. (2016). From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Annals of the New York Academy of Sciences, 1417(1), 5–22. doi:10.1111/nyas.13011
- ↑ https://ghr.nlm.nih.gov/gene/FOXP3#conditions
- ↑ Ziegler, S.F. 2006. FOXP3: of mice and men. Annu. Rev. Immunol. 24: 209–226.
- ↑ L. A. Schubert, E. Jeffery, Y. Zhang, F. Ramsdell, and S. F. Ziegler, “Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation,” Journal of Biological Chemistry, vol. 276, no. 40, pp. 37672–37679, 2001.
- ↑ Y. Wu, M. Borde, V. Heissmeyer, et al., “FOXP3 controls reg- ulatory T cell function through cooperation with NFAT,” Cell, vol. 126, no. 2, pp. 375–387, 2006.
- ↑ Bacchetta, R., Barzaghi, F., & Roncarolo, M.-G. (2016). From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Annals of the New York Academy of Sciences, 1417(1), 5–22. doi:10.1111/nyas.13011
- ↑ Rubio-Cabezas, O., Minton, J. A. L., Caswell, R., Shield, J. P., Deiss, D., Sumnik, Z., … Hattersley, A. T. (2008). Clinical Heterogeneity in Patients With FOXP3 Mutations Presenting With Permanent Neonatal Diabetes. Diabetes Care, 32(1), 111–116. doi:10.2337/dc08-1188
- ↑ Bacchetta, R., Barzaghi, F., & Roncarolo, M.-G. (2016). From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Annals of the New York Academy of Sciences, 1417(1), 5–22. doi:10.1111/nyas.13011
- ↑ R. S. Wildin, S. Smyk-Pearson, and A. H. Filipovich, “Clinical and molecular features of the immunodysregulation, polyen- docrinopathy, enteropathy, X linked (IPEX) syndrome,” Jour- nal of Medical Genetics, vol. 39, no. 8, pp. 537–545, 2002.
- ↑ O. Baud, O. Goulet, D. Canioni, et al., “Treatment of the immune dysregulation, polyendoccrinopathy, enteropathy, X- linked syndrome (IPEX) by allogeneic bone marrow trans- plantation,” The New England Journal of Medicine, vol. 344, no. 23, pp. 1758–1762, 2001.
- ↑ M.L.Heltzer,J.K.Choi,H.D.Ochs,K.E.Sullivan,T.R.Torg- erson, and L. M. Ernst, “A potential screening tool for IPEX syndrome,” Pediatric and Developmental Pathology, vol. 10, no. 2, pp. 98–105, 2007.
- ↑ Gambineri, E., L. Perroni, L. Passerini, et al. 2008. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J. Allergy Clin. Immunol. 122: 1105–1112.e1.
- ↑ Burroughs, L.M., T.R. Torgerson, R. Storb, et al. 2010. Stable hematopoietic cell engraftment after low‐intensity nonmyeloablative conditioning in patients with immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome. J. Allergy Clin. Immunol. 126: 1000–1005.
- ↑ Bacchetta, R., Barzaghi, F., & Roncarolo, M.-G. (2016). From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Annals of the New York Academy of Sciences, 1417(1), 5–22. doi:10.1111/nyas.13011
Additional information
This page was developed for the course on Structural biology of the cell at Charles University.