We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Group:MUZIC:actinin2
From Proteopedia

| |||||||||||
References
- ↑ Ribeiro Ede A Jr, Pinotsis N, Ghisleni A, Salmazo A, Konarev PV, Kostan J, Sjoblom B, Schreiner C, Polyansky AA, Gkougkoulia EA, Holt MR, Aachmann FL, Zagrovic B, Bordignon E, Pirker KF, Svergun DI, Gautel M, Djinovic-Carugo K. The Structure and Regulation of Human Muscle alpha-Actinin. Cell. 2014 Dec 4;159(6):1447-60. doi: 10.1016/j.cell.2014.10.056. Epub 2014 Nov, 26. PMID:25433700 doi:http://dx.doi.org/10.1016/j.cell.2014.10.056
- ↑ Sanger JW, Kang S, Siebrands CC, Freeman N, Du A, Wang J, Stout AL, Sanger JM. How to build a myofibril. J Muscle Res Cell Motil. 2005;26(6-8):343-54. PMID:16465476 doi:10.1007/s10974-005-9016-7
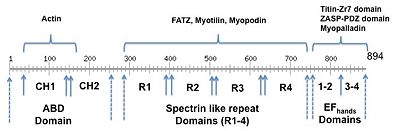
- ↑ 3.0 3.1 Franzot G, Sjoblom B, Gautel M, Djinovic Carugo K. The crystal structure of the actin binding domain from alpha-actinin in its closed conformation: structural insight into phospholipid regulation of alpha-actinin. J Mol Biol. 2005 Apr 22;348(1):151-65. PMID:15808860 doi:http://dx.doi.org/10.1016/j.jmb.2005.01.002
- ↑ Faulkner G, Pallavicini A, Formentin E, Comelli A, Ievolella C, Trevisan S, Bortoletto G, Scannapieco P, Salamon M, Mouly V, Valle G, Lanfranchi G. ZASP: a new Z-band alternatively spliced PDZ-motif protein. J Cell Biol. 1999 Jul 26;146(2):465-75. PMID:10427098
- ↑ Au Y, Atkinson RA, Guerrini R, Kelly G, Joseph C, Martin SR, Muskett FW, Pallavicini A, Faulkner G, Pastore A. Solution structure of ZASP PDZ domain; implications for sarcomere ultrastructure and enigma family redundancy. Structure. 2004 Apr;12(4):611-22. PMID:15062084 doi:10.1016/j.str.2004.02.019
- ↑ Fukami K, Sawada N, Endo T, Takenawa T. Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle alpha-actinin. J Biol Chem. 1996 Feb 2;271(5):2646-50. PMID:8576235
- ↑ Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992 Sep 10;359(6391):150-2. PMID:1326084 doi:http://dx.doi.org/10.1038/359150a0
- ↑ Young P, Gautel M. The interaction of titin and alpha-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism. EMBO J. 2000 Dec 1;19(23):6331-40. PMID:11101506 doi:10.1093/emboj/19.23.6331
- ↑ Ylanne J, Scheffzek K, Young P, Saraste M. Crystal structure of the alpha-actinin rod reveals an extensive torsional twist. Structure. 2001 Jul 3;9(7):597-604. PMID:11470434
- ↑ Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995 Aug 15;92(4):785-9. PMID:7641357
- ↑ Chiu C, Bagnall RD, Ingles J, Yeates L, Kennerson M, Donald JA, Jormakka M, Lind JM, Semsarian C. Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis. J Am Coll Cardiol. 2010 Mar 16;55(11):1127-35. doi: 10.1016/j.jacc.2009.11.016. PMID:20022194 doi:10.1016/j.jacc.2009.11.016
Proteopedia Page Contributors and Editors (what is this?)
Euripides Ribeiro, Alexander Berchansky, Nikos Pinotsis, Michal Harel, Jaime Prilusky