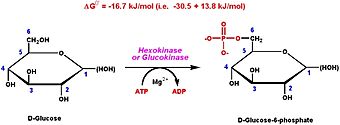
Hexokinase Type I is one of four hexokinase isoenzymes that participate in glycolysis. Hexokinase catalyzes the phosphorylation of glucose into glucose-6-phosphate (G6P) providing enough activation energy for the glycolytic process to start. Thus hexokinase allows the muscle cells to take up glucose in the blood and use it as an energy source for different actions and expenditures.[2]
Hexokinase Type I is considered an enzyme of maintenence; it is unaffected by most physiological, metabolic and hormonal changes within the biological system.[3] Hexokinase Type I is mainly found in mammalian tissues; it is crucial enzyme in maintaining a downward concentration gradient to provide a steady influx of glucose into cells.[2]
Introduction
Four types of hexokinase isozymes exist in the human biological system. They all serve to catalyze the exact same reaction in glycolysis even though they are encoded by different sets of genes. The hexokinase types I-III all have a high affinity for glucose and become subject to inhibition in the presence of glucose-6-phosphate- the first step reaction product in glycolysis.[2] Hexokinase type I is the predominant form in the muscle while hexokinase type II is the predominant form in myocytes. Hexokinase type IV varies from the other three hexokinase types as it is not inhibited by the production of glucose-6-phosphate and shows a lower affinity for glucose. Hexokinase type I and II have the particular ability to bind to the outer mitochondrial membrane, this binding happens both specifically and reversibly.[4]
Structural Overview
The size of hexokinase type I is approximately 100 kD.[5] Hexokinase type I is constructed by a N-terminal regulatory domain and a C-terminal catalytic domain joined together by an alpha helix. The glucose binding site of hexokinase type I can be found within the two sub-units that make up the isoenzyme, these are known as lobes. Factors that contribute to the binding of glucose to this active site include amino acids within the actual site and hydrogen bonding that takes place on the glucose between the hydroxyl groups.[6]
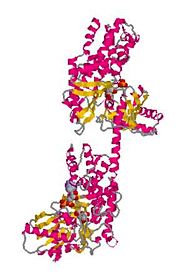
Figure 2:The C-terminal and N-terminal regions of Hexokinase Type I - held together by hydrogen bonding and a connecting helix.
[7] Glucose Binding Sites
The residues of the glucose binding site of Hexokinase Type I are very highly conserved within the hexokinase sequence; glucose binds equally to both domains or "lobes" of the structure. As a result, hexokinase type I in its native conformation has an active site in its inactive regulatory domains. Before glucose binds to hexokinase type I, it is said to be in an open conformation. ATP is already bound within one of the domains but it is situated a distance from the glucose binding site. As the glucose binds, the two domains close around the glucose substrate and this change results in a newly formed pattern of hydrogen bonding.[4]
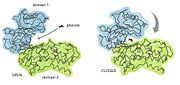
Figure 3:The conformational change in hexokinase caused by glucose binding.
[8] Active Site
In the of hexokinase type I, -denoted in red, and -denoted in blue, show the hydrogen-bonding distance in which a reactive O6 hydroxyl is situated in between. Here Lys 621 functions to aid the transfer of the phosphate moiety which is negatively charged; Asp 657 serves as a catalytic base or to position the glucose O6 correctly for phosphoryl transfer to take place.[4] Though it may seem that
-denoted in black, may have functional significance, there has been no observed interaction of Ser 603 with glucose substrates. A torsional rotation of of the hydroxyl group brings about a much better distance for hydrogen bonding of the glucose O6. As a result of this observation, it can be assumed that Ser 603 may have some transient role during phosphorylation.[4]
Regulatory Binding Site
As glucose-6-phosphate is being produced it binds to either one of the domains on hexokinase type I. On domain 1, the phosphate moiety is surrounded by . The presence of these three residues generate an anion binding site that is approximately 5.6 Å to the 6 hydroxyl of the glucose that is bound.[4]
Functional Overview
Basic Function
Hexokinase Type I functions in a mainly catabolic role; it is responsible for introducing glucose the glycolytic process in attempts to produce ATP. Hexokinase Type I phosphorylates a hexose into a hexose phosphate; most commonly the substrate of hexokinase I is found to be glucose and the product found to be glucose-6-phosphate.[9] Mammalian brain tissue shows a high content of Hexokinase Type I which reiterates the idea that this isoenzyme is needed to maintain high rates of energy metabolism. Hexokinase Type I associated with brain homogenates demonstrates an interaction with outer mitochondrial membrane. The binding of to this mitochondria is highly dependent on the N-terminus sequence. The protein porin is then responsible for the formation of a channel in which metabolites can pass through the mitochondrial membrame.[10]
Allosteric Regulation
Entry of glucose into a cell is highly dependent on the number of glucose transporters present on the cell surface and the affinity that these specific transporters have for glucose. Expression of these glucose transporter members vary in level and strength different from tissue to tissue. Hexokinase type I has a low Km and therefore a high affinity for glucose. This allows the initiation of glycolysis even when blood glucose levels are relatively low. The inhibition of hexokinase type I is caused by its product, glucose-6-phosphate. This inhibitory step prevents over-consumption of cellular ATP when glucose is not limiting. [11]
Mechanism of Inhibition and Relief
The activity of hexokinase type I becomes inihibited when glucose-6-phosphate binds to the inactive, N-terminal half of the enzyme. This binding stimulates the inhibition of the active, C-terminal half and as a result, the catalysis of glycolysis becomes stagnant. The presence of orthophosphate relieves the product inhibition by displacing the bound glucose-6-phosphate and binding to the enzyme itself. The N-terminal half then responds differently to the newly bound orthophosphate and evokes an active reaction from the C-terminal half. The ability of the C-terminal half to significantly differentiate between the two possible binding molecules is evident by the presence of ADP-ligated active sites on the hexokinase type I enzyme as well as ADP binding sites on the N-terminal halves.[2]
3D structures of hexokinase I
See Hexokinase
Additional Resources
For additional information, see: Carbohydrate Metabolism