A Cryo-Crystallographic Time Course for Peroxide Reduction by Rubrerythrin from Pyrococcus furiosus
Bret Dillard, Jonathan Demick, Michael Adams and William Lanzilotta[1]
Molecular Tour
Oxidative damage is a major problem for all organisms and not surprisingly a number of protection systems exist in cells across all kingdoms of life. For organisms that respire using anaerobic processes oxidative damage can be an even more pressing problem as these cells may be destroyed by the simple presence of molecular oxygen. It is not that surprising that strict anaerobes have developed a system for the rapid reduction and removal of molecular oxygen in two steps. The first involves reduction of oxygen to peroxide followed by peroxide reduction to water. The terminal enzyme in this systems, rubrerythrin, has been well characterized in a number of bacteria that live at ambient temperatures. In this work, we report the first time course that monitors peroxide reduction by a rubrerythrin isolated from an organism that lives at an optimal temperature of one-hundred degrees centigrade. The slow turnover of the enzyme at room temperature has allowed us to trap intermediates and track the atomic details of this important enzyme reaction.
is formed by 2 adjacent monomers A (colored darkmagenta) and B (colored magenta). Rubrerythrin in the (3pwf). Rubrerythrin in the (3mps), after 20 s exposure to peroxide, has μ-oxo bridge. Monomers of oxidized rubrerythrin A (colored skyblue) and B (colored cyan). This exposure . Movement of E114 upon oxidation of the all-ferrous diiron center to the fully oxidized all-ferric
state results in a . The in some cases. reveals (3pza). also reveals (3qvd). Both these exposures identified previously unobserved intermediates in the reaction cycle.
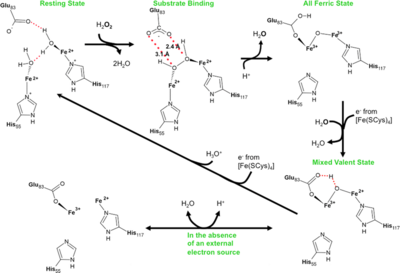
Reaction scheme for rubrerythrins.
PDB reference: Peroxide Bound Oxidized Rubrerythrin from Pyrococcus furiosus, 3mps; High resolution structure of the fully reduced form of rubrerythrin from P. furiosus, 3pwf; Fully Reduced (All-ferrous) Pyrococcus rubrerythrin after a 10 second exposure to peroxide, 3pza; Exposure of rubrerythrin from Pyrococcus furiosus to peroxide, fifteen second time point, 3qvd.


