Crystallographic studies of [NiFe]-hydrogenase mutants: towards consensus structures for the elusive unready oxidized states
Anne Volbeda, Lydie Martin, Elodie Barbier, Oscar Gutierrez-Sanz, Antonio L. De Lacey, Pierre-Pol Liebgott, Sebastien Dementin, Marc Rousset, Juan Fontecilla-Camps [1]
Molecular Tour
(small subunit is in cyan and large subunit is in magenta) attract much interest because of their ability to cleave or produce the chemical bond in the simplest of energy carriers, molecular hydrogen. A major problem for potential biotechnological applications of most of these enzymes is their inactivation by molecular oxygen. In order to study the chemical processes responsible for the formation of unready, difficult to activate oxidized states of such oxygen-sensitive [NiFe]-hydrogenases, this paper describes a detailed spectroscopic and structural analysis of enzyme mutants with special properties. So far, progress in the fundamental understanding of the reactions with molecular oxygen was limited by problems of structural heterogeneity resulting from the very rich redox chemistry of the active site of these enzymes. In addition, the oxidized unready (difficult to activate) states were difficult to characterize by X-ray crystallographic methods because of the reducing effects of X-ray induced photoelectrons. This study shows how the oxygen-sensitive [NiFe]-hydrogenase of the sulfate-reducing bacterium Desulfovibrio fructosovorans reacts, under air, with oxygen and metabolic sulfur, forming oxidized thiolate ligands. In one mutant no reaction with sulfur takes place and a pure unready .
The atoms are colored according to the CPK Color Scheme: C
O
N
S
Fe
Ni, except for amino acids carbon atoms, which are in magenta, dashed line indicates possible H-bonding interaction.
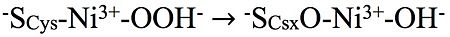
Chemical reaction producing the Ni-A state from a transient peroxide intermediate
Another mutant is especially sensitive to sulfur and is characterized in an unready enzyme mixture consisting of the Ni-A state and a new . The atoms are colored according to the CPK Color Scheme: C
O
N
S
Fe
Ni, except for amino acids carbon atoms, which are in darkmagenta.
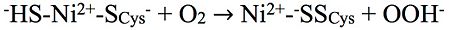
Chemical reaction producing the Ni-\'Sox\' state'
The reactivity of the Ni-Fe site to sulfur complicated the interpretation of previous crystallographic studies. The new results presented here should conclude a long-standing debate on the identity of the Ni-A state. A proper understanding of the reactions of the enzyme Ni-Fe active site with molecular oxygen and inorganic sulfur may also have an impact on the design and synthesis of bioinspired synthetic catalysts.
PDB references: Structure of the unready Ni-A state of the S499C mutant of D. fructosovorans NiFe-hydrogenase, 4upe; Low X-ray dose structure of a Ni-A Ni-Sox mixture of the D. fructosovorans NiFe-hydrogenase L122A mutant, 4upv; High-resolution structure of a Ni-A Ni-Sox mixture of the D. fructosovorans NiFe-hydrogenase L122A mutant, 4uql; High-resolution structure of the D. fructosovorans NiFe-hydrogenase L122A mutant after exposure to air, 4uqp; High-resolution structure of partially oxidized D. fructosovorans NiFe-hydrogenase, 4urh.

