The extracellular subunit interface of the 5-HT3 receptors: a computational alanine scanning mutagenesis study
Francesca De Rienzo, Arménio J. Moura Barbosa, Marta A.S. Perez, Pedro A. Fernandes, Maria J. Ramos, Maria Cristina Menziani [1]
Molecular Tour
The serotonin type-3 receptor (5-HT3-R) is a cation selective transmembrane protein channel that belongs to the Cys–loop Ligand-Gated Ion Channel (LGIC) superfamily (http://www.ebi.ac.uk/compneur-srv/LGICdb/LGICdb.php), which also includes receptors for nicotinic acetylcholine (, PDB code 2bg9), γ-aminobutyric acid and glycine. 5-HT3-R is involved in signal transmission in the central and peripheral nervous system and its malfunctioning leads to neurodegenerative and psychiatric diseases, therefore it is an important target for drug design research. A few drugs active against 5-HT3-R are already on the market, such as, for example, palonosetron (http://en.wikipedia.org/wiki/Palonosetron) and granisetron (http://en.wikipedia.org/wiki/Granisetron).
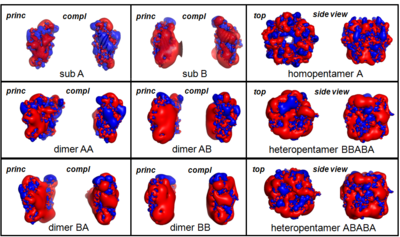
The 5-HT3R is made of five monomers assembled in a to form an ion channel permeable to small ions (Na+, K+); each subunit contains three domains: an (shown on the example of nAChR, 2bg9). To date, five different 5-HT3-R subunits have been identified, the 5-HT3 A, B, C, D and E; however, only subunits A and B have been extensively characterised experimentally. The of nAChR is located at the extracellular region, at the interface between two monomers (α-γ and α-δ; 2 identical α monomers, chains A and D, are colored in same color - lavender), called the principal and the complementary subunits.
The 3D structure of 5-HT3-R has not been experimentally solved yet; however, it has been obtained computationally by means of homology modelling techniques. (http://salilab.org/modeller/)
Thus, the are modelled by homology with the 3D structure of the nAChR subunit A (2bg9-A) and are used to assemble receptor structures as pseudo-symmetric pentamers made either of or of in a still debated arrangement.[2] Subunits A and B are colored in magenta and red, respectively.
A complete characterization of the extracellular moiety of the (AA dimer is shown, principal subunit is colored in cyan and complementary is in blue, is obtained by the Computational Alanine Scanning Mutagenesis (CASM) approach [3], which simulates the substitution, one by one, of all the amino acid residues at the subunit-subunit interfaces with an Ala, thus to assess the interface binding contribution of single residue side-chains. The are classified as “hot spots” that stabilize the interface by more than 4 kcal/mol and “warm spots” that contribute to interface stabilization by more than 2 kcal/mol. Interface residues are shown in spacefill representation, hot spot residues are colored in red and warm spots residues are are in orange.
From this analysis the located at the interface core and formed by residues W178 (principal subunit), Y68, Y83, W85 and Y148 (complementary subunit) is highlighted.[4] In addition, two important groups of interface residues are probably involved in the coupling of and binding to channel activation/inactivation: W116-H180-L179-W178-E124-F125 (principal subunit) and Y136-Y138-Y148-W85-(P150) (complementary subunit), where W178 and Y148 appear to be critical residues for the binding/activation mechanism. Finally, the (principal subunit of AA is colored in cyan, principal subunit BB is colored in darkmagenta, complementary subunit AA is in blue and complementary subunit BB is in magenta) shows differences which could explain the reasons why the homopentamer 5-HT3B-R, if expressed, is not functional (see also image below).[5]