We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
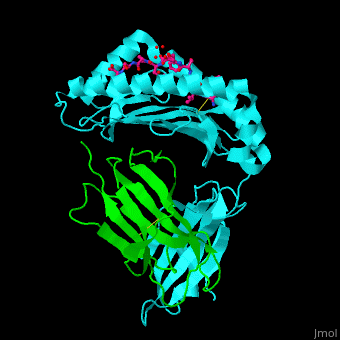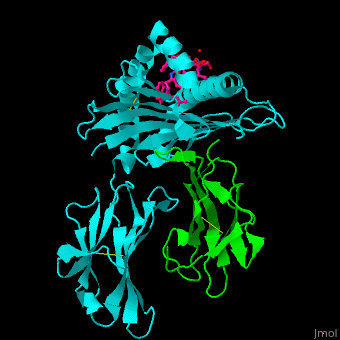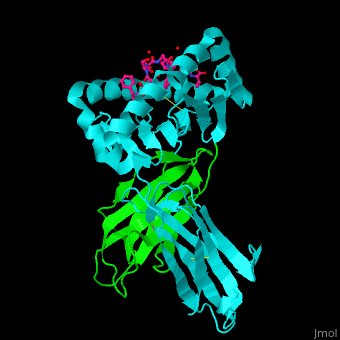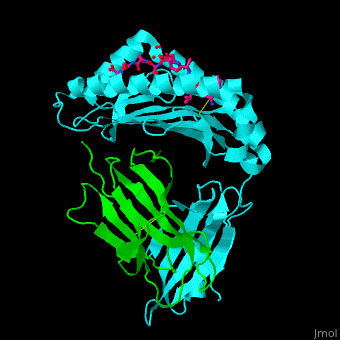
Major histocompatibility complex
From Proteopedia
| |||||||||||
See Also
- Major Histocompatibility Complex Class I which is about the history and impact of the first crystal structure.
- A narrated YouTube video tutorial on MHC I and II (25 min) available at MolviZ.Org
- Highest impact structures: 1987.
References
- ↑ Raghavan M, Del Cid N, Rizvi SM, Peters LR. MHC class I assembly: out and about. Trends Immunol. 2008 Sep;29(9):436-43. doi: 10.1016/j.it.2008.06.004. PMID:18675588 doi:http://dx.doi.org/10.1016/j.it.2008.06.004
- ↑ Miller MS, Douglass J, Hwang MS, Skora AD, Murphy M, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S, Gabelli SB. An engineered antibody fragment targeting mutant beta-catenin via Major Histocompatibility Complex I neoantigen presentation. J Biol Chem. 2019 Nov 5. pii: RA119.010251. doi: 10.1074/jbc.RA119.010251. PMID:31690625 doi:http://dx.doi.org/10.1074/jbc.RA119.010251
- ↑ Holling TM, Schooten E, van Den Elsen PJ. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum Immunol. 2004 Apr;65(4):282-90. PMID:15120183 doi:http://dx.doi.org/10.1016/j.humimm.2004.01.005
- ↑ Liu J, Chen KY, Ren EC. Structural insights into the binding of hepatitis B virus core peptide to HLA-A2 alleles: Towards designing better vaccines. Eur J Immunol. 2011 Jul;41(7):2097-106. doi: 10.1002/eji.201041370. PMID:21538979 doi:10.1002/eji.201041370
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, Tihitina Y Aytenfisu, Eric Martz, Sandra B. Gabelli