MUTATIONS OF PTEN IN CANCER
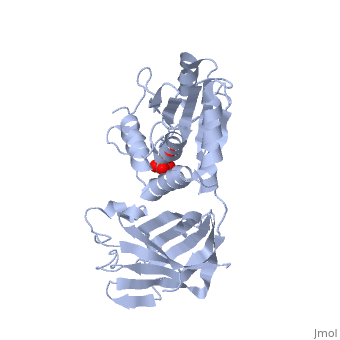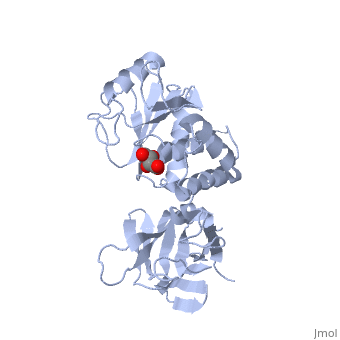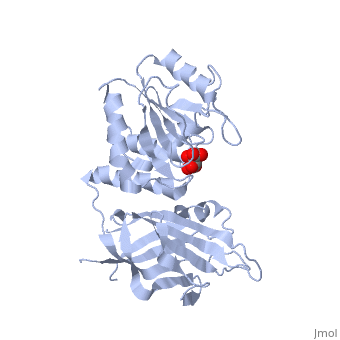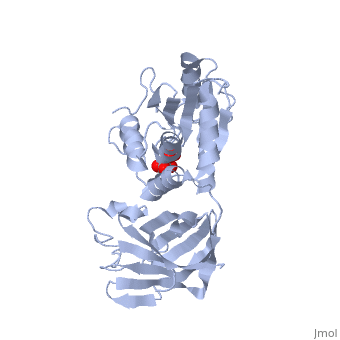
PTEN is a tumor suppressor protein that is mutated in several human cancers. These include glioblastomas, endometrial carcinomas, prostate carcinomas, and melanoma cases. This protein is a phosphatase that acts on both polypeptide and phosphoinositide substrates [[1]]. PTEN has 403 amino acids which are separated into The red section represents the C2 domain which allows the protein to bind with phospholipid membrane. The phosphatase section (blue) interacts with the ligand and the phosphate head. These interactions are controled by two separate loops. The is responsible for the catalytic reactions performed by the protein. These reactions are what halt cell growth. The P-loop is made up of two parts, the catalytic parts (green) that perform the reactions and the conformational parts (purple) that force the loop into its conformational shape. The other loop is the This loop interacts with the lipid while in the active P-loop to increase the catalysis.
Mutations of PTEN occur within of the these different areas of the protein. The P-loop itself contains three mutations all of which decrease the protein's activity. This results in excessive cells growth and tumors. The amino acid mutated in the loop are , which decrease the protein's activity from 50-60%. The "TI" loop also contain mutations. These include mutations of Though the Gln171 mutation is not directly inside the "TI" loop, its close proximity does affect the loop's function. These mutations decrease the activity by 60% and 75%. Even though this loop is not a part of the catalysis, its decreased interactions with the lipid hinders the catalytic reactions of the P-loop. In addition to the active loops in the phosphatase domain, the C2 domain also contains mutations that affect the protein's ability to interact with the membrane. This mutation is located at the which is mutated to a Gln. There are several mutations located along some of the general portions of the protein. The most influencial of of these is the mutation of to Ala. This mutation alone causes a 700-fold reduction in the protein's catalytic activity. The last set of general mutations are involved in the interdomain hydrogen bonding the takes place between the two domains. Of the several amino acids that are incorporated into the hydrogen bonding, are two of the eight most frequently mutated residues of PTEN. The severe decrease in phosphatase activity due to these mutations confirms the extreme importance of this interface for PTEN function.
Additional Resources
For Additional Information, See:
REFERENCES
- Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999 Oct 29;99(3):323-34. PMID:10555148