PGK in the Glycolysis Cycle
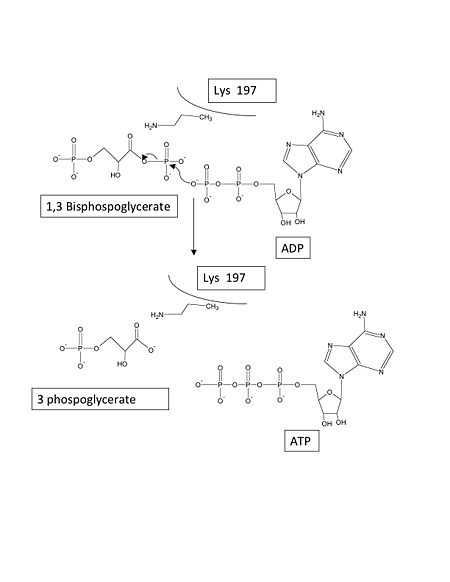
Phosphoglycerate kinase is a crucial enzyme in the glycolysis cycle. This cycle is a series of ten reactions which ultimately breaks down glucose into pyruvate while generating 2 NADH and 2 ATP molecules. Phosphoglycerate kinase is the seventh enzyme in the cycle which catalyzes the reaction of 1,3-Biphosphoglycerate and ADP to produce and . This method for ATP production is known as substrate-level phosphorylation because it produces energy storing ATP molecules without the use of oxygen, NADH, or an ATPase. The reaction is highly exergonic allowing it to be coupled with the less thermodynamically favored GADPH reaction of the cycle so both reactions occur spontaneously. See Glycolysis Enzymes, Gluconeogenesis.
Structure
The overall structure of Phosphoglycerate kinase is very distinctive. It is a monomeric protein consisting of approximately 400 amino acids, with a molecular weight of about 45kD[1]. The structure is distinctly bilobed with a depressed region between the two lobes or domains. The lobes/domains are clearly connected at only two locations: . The SCOP classification of PGK is alpha and beta, indicating that its is composed of roughly equal numbers alpha and beta sheets.
PGK structure shows an open-to-close transition upon hinge bending. PG assumes the open conformation upon release of PGA and ATP. The closed conformation active site contains PGA, ADP and AlF4-1 ion which mimics the phosphate ion[2].
- .
- .
- .
- . Water molecules are shown as red spheres.
Reaction Mechanism
The bilobed structure of PGK is very crucial in its catalytic function. The active site is broken into two pieces, one on the interior of each lobe or domain. The N-terminal domain has a basic region where the 1,3-Biphosphoglycerate and 3-phosphoglycerate bind while the C-terminal domain has the binding sites for the nucleotide substrates, ADP and ATP. Upon binding of both substrate molecules at the active sites, the protein’s conformation changes such that the two lobes of the protein swing together [3] When the two domains swing shut, a hydrophobic chamber free from water is established where the reaction can take place. This hydrophobic chamber is necessary to prevent ATP hydrolysis [1] The hinge for this conformational change is beta sheet L and the new conformation is formed via a salt bridge between [4] Recent research indicates that the mechanism for closure of the two domains is a series of hydrogen bond interactions that occur upon binding of the substrates on both domains [5]
The mechanism of catalysis has not been fully established because the PGK/1-3biphophoglycerate complex is highly unstable; however, it is thought that the mechanism is similar to that of hexokinase. Hexokinase catalyzes the removal of a phosphate group from ATP to glucose and has a very similar structure and conformational change via a hinge. PGK has a similar function except it catalyzes the transfer of a phosphate to form ATP instead of using ATP. The reaction of PGK removes the C1 phosphate group from 1,3-biphosphoglycerate and transfers it to ADP to form ATP. Once the substrates bind to the active sites, the protein domains swing shut forcing the substrates into correct position for the reaction to proceed[6].
The general mechanism is a single displacement Sn2 reaction in which the ADP-B-phosphate oxygen atom initiates nucleophilic attack on the 1-phosphate group of 1-3biphosphoglycerate [1]. Thus, the phosphoryl group is transferred directly via a charged transition state. The product, ATP, is favored because it's negatively charged oxygens of the 3 phosphates form with the enzyme. The 3 hydrogen bonds of ATP are favored over the 2 hydrogen bonds of ADP.
Two specific residues known to be necessary for catalysis are . Lys 197 secures 1,3-biphosphoblycerate in the closed conformation, and it has been proposed that the transition state intermediary is stabilized by the highly conserved Lys 197 as it transfers the phosphate group. Additionally, it has been shown that Arg 38 is also necessary for catalytic function. Arg 36 has been shown to stabilize a water molecule in the closed conformation and may form a hydrogen bond with the ATP product [1].
Kinetics
Given that Phosphoglycerate kinase is a monomeric protein standard Michealis-Menton kinetics would be expected; however, this is not the case. Multiple experiments have shown that the data, when transformed into either double-reciprocal or Eadie-Hofstee plots is non-linear; Eadie-Hofstee plots curve upward. One possible explanation for the non-linearity, negative cooperativity, is ruled out because PGK does not have multiple subunits. In one study that conducted kinetic tests with a 1000 fold range of substrates, at the highest concentrations of substrate the rate was still increasing; this puts the Km value in the 2-5mM range [7]. Recently, a new model was proposed to explain this conflict between the seemingly negative cooperative kinetics and the monomeric structure of PGK. The enzyme may form a complex with the metabolic enzyme glyceraldehyde-3-phosphate dehydrogenase. This multi-subunit complex would be capable of the negative cooperativity that is indicated by the non-linear kinetics of PGK[8].
Regulation
The role of PGK in glycolysis is very important for the production of ATP through substrate level phosphorylation. Regulation of this protein is thereby controlled by ATP or energy level of the cell. Recent research in frogs which can withstand freezing temperatures indicates that PGK is up regulated by the cold and more specifically by low levels of oxygen[9]. This makes logical sense as when oxygen is low, oxidative phosphorylation cannot occur. Thus without oxygen and oxidative phosphorylation, ATP levels begin to drop. In response to decreased ATP, PGK is up regulated to increase the amount of ATP produced by substrate level phosphorylation. It could therefore also be expected that ADP might act to inhibit or down regulated PGK expression.
Other Functions
Recent study of PGK has revolved around its function in tumor formation and growth. It has been shown that in addition to catalyzing its normal reaction of 1,3-Biphosphoglycerate and ADP to ATP and 3-Phosphoglycerate, PGK can also function to cleave disulfide bonds. Specifically, the review of sulfide bond cleavage indicates PGK has been shown to cleave disulfide bonds in the protein zymogen plasmin to produce the active form of the protein. The active form of plasmin is responsible for angiogenesis or blood vessel formation in tumors[10] Without the formation of blood vessels in tumors, nutrients are limited and tumor growth is therfore limited. Once blood vessels are established growth can rapidly increase. The fact that tumor cells secrete PGK to allow blood vessel formation through the activation of the zymogen plasmin has important implications for understanding its regulation. If the regulation of PGK in tumor cells can be understood, it might be possible to inhibit the overproduction and secretion of PGK to limit angiogenesis in tumors.
3D structures of phosphoglycerate kinase
Phosphoglycerate kinase 3D structures