Function
Potassium Channels control cell membrane electric potentials by selectively allowing diffusion of K+ across the membrane.[1] K+ Channels extend across the cell membrane, a 40Å thick lipid bilayer which ions cannot cross.[2] Potassium homeostasis is crucial for nearly all living cells, but is particularly important for the correct function of neurons. Neurons produce electrical impulses known as action potentials, allow for cellular communication processes like neurotransmitter release or to initiate intercellular processes muscle contraction. At the onset of an action potential, sodium ions flood across the plasma membrane of neurons via sodium channels. The change in polarity of the plasma membrane caused by the sodium ion influx inactivates sodium channels. Potassium channels subsequently open allowing the selective diffusion of K+ ions across the plasma membrane, returning the membrane polarity to neutral. After the action potential has passed, channels recreate the high potassium concentration within the cell in preparation for the next stiumulus.[3]
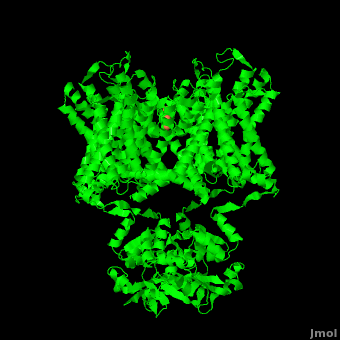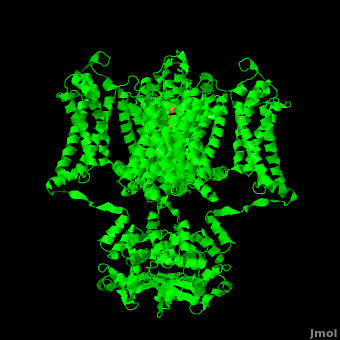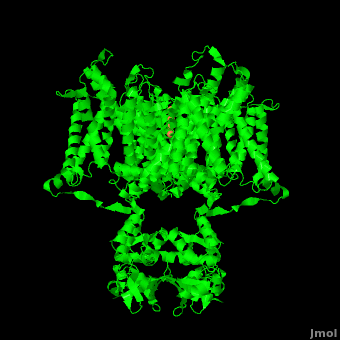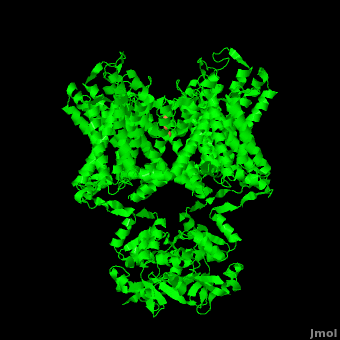
Potassium channels possess two traits that are seemingly mutually exclusive. Firstly, potassium channels have exquisite selectivity, with an amazing 10,000 fold selectivity for K+ ions over sodium ions. Considering the only difference by which potassium ions can be differentiated from sodium ions is potassium ions’ 1.33Å Pauling radius vs. Sodium’s .95Å radius, the selectivity of potassium channels is remarkable.[2] Second, despite its remarkable selectivity, potassium channels allow for the transfer of K+ ions across the cell membrane at a rate of nearly 108 per second, nearly at the diffusion rate limit.[4] Potassium channels are able to achieve these remarkable feats due to its amazing structural architecture, which contains several features which not only can sense the voltage potential across a membrane, but also selectively ferry K+ ions without any outside energy expenditure.
The overall structure of the voltage gated potassium channel can be seen in the image at the left. It is comprised of 4 identical subunits and contains several key features which will be analyzed. Primarily, a marked between the parallel lines in the figure. This region houses the , composed of interwoven helices in a teepee conformation, the all-important , providing the channel with its remarkable 10,00 fold selectivity for K+ ions over Na+ ions and the which is uses well placed arginine and acidic residues to determine the membrane polarity and open/close the channel in response.[4]
Disease
Mutations in voltage-gated potassium channel KCNC3 have been linked with neurodevelopmental disorders and neurodegeneration.[5]. Amiodarone is a potassium channel blocker used in treatment of cardiac dysrhythmias.
Selectivity Filter and Pore
It is instructive to follow the path of a potassium ion as it enters the cell through the . Upon , the K+ ion first comes into contact with the . The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the , K+ ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, () bind the K+ ions. The distance between K+ ion and carbonyl oxygen is at to accommodate K+ ions but not Na+, ions which are too small. If a Na+ ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na+ ion to enter the channel. There is room within the selectivity filter for . This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.[2] Also, the , with the , helps pull the positively charged ions through the channel quickly. Compared to the (1k4c), when exposed to a low concentration of potassium, the channel assumes a (1k4d) which is sealed shut via interactions with water molecules.[1]
The only makes up the beginning of the . With the exception of the selectivity filter, the pore lining is . This hydrophobic lining provides an inert surface over which the diffusing ion can slide unimpaired. Immediately following the selectivity filter is an (). K+ ions, after passing through the filter, rehydrate in this cavity, helping overcome much of the energetic difficulty of having a positively charged cation within a hydrophobic membrane. At the bottom of the 34Å pore containing transmembrane region lies a number of which help form a seal between the pore and the intracellular cytoplasm.[2]
Voltage Sensor
Channel pore opening is dependent on the membrane voltage, a characteristic that is “sensed” by the . The voltage sensor is comprised of , S0, S1, S2, S3, S4, & S5. Negatively charged sensor residues are either located in the , consisting of Glu 183 (in the 2r9r structure) and Glu 226, or in the consisting of Glu 154, Glu 236, and Asp 259. The external cluster is exposed to solvent while the internal cluster is buried. acts as a separator between the two clusters.[4] The 7 of the voltage sensor are located on the S4 helix. Lys 302 and Arg 305 with the internal negative cluster while Arginines 287, 290, 293, 296 and 299 are (). When the voltage sensor is exposed to a strong negative electric field in the intracellular membrane, the positive gating charges shift inward with the α-carbon of Arg 290 coming in close proximity to Phe 233. This shift effectively squeezes the pore shut, closing the intracellular-extracellular pathway. For a comparison see: The Channel vs. The (1k4c) Channel.[4] Or view the morph of the ().
Overall, Potassium channels are remarkable structures that allow for near diffusion limit transfer of molecules with sub-angstrom specificity. Our understanding of the structure of Potassium Channels has opened up the potential for therapeutic intervention into Potassium channel related diseases.
See also Potassium channel Xavier
Eag domain-CNBHD complex of the mouse EAG1.
Page Development
This article was developed based on lectures given in Chemistry 543 by Prof. Clarence E. Schutt at Princeton University.
Potassium channels (KCh) are subdivided into voltage-gated KCh and calcium-dependent KCh. The latter are subdivided into high- (BK, LKCa), intermediate- and small-conductance KCh (human SK1, rat SK2, SKCa). The T1 domain is a highly conserved N-terminal domain which is responsible for driving the tetramerization of the KCh α subunit. The inward rectifier KCh (IRK) passes current more easily in the inward direction. KCh is activated by Phosphatidylinositol bisphosphate (PIP2). MthK is a calcium-dependent KCh from Methanobacterium thermoautrophicum.
3D structures of Potassium Channels
Potassium channel 3D structures