Function
Prion (PrP) is a protein which becomes infectious upon undergoing conformation change to an amyloid form, which is self-propagating and becomes resistant to protease degradation. The fungus Podospora anserine has a prion-like protein HET-S which undergoes a conformation change to amyloid form which prevents its colony from merging with non-compatible colonies. Yeast prion proteins are Sup35 and Ure2. For more details see
Click here to see (morph was taken from Gallery of Morphs of the Yale Morph Server).
Dominant-negative Effects in Prion Diseases
Insights from Molecular Dynamics Simulations on [1].
The key event in prion diseases is the conformational conversion from the cellular form of the prion protein (PrPC) to its pathogenic scrapie form PrPSc (or prion). PrPSc is the sole causative agent of prion diseases which self-propagates by converting PrPC to nascent PrPSc. Mutations in the open reading sequence of the prion protein gene can introduce changes in the protein structure and alter PrPSc formation and propagation, possibly by (de)stabilizing the physiological folding of PrPC and/or affecting its interactions with some yet unknown cellular factors. Some PrP polymorphisms may even inhibit the wild-type (WT) PrPC from being converted to PrPSc, with the so-called “dominant-negative” effect.
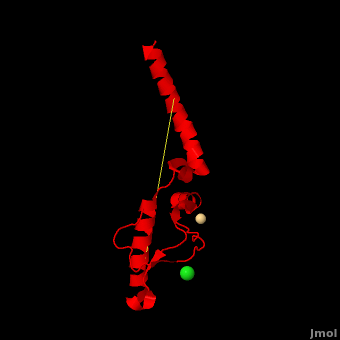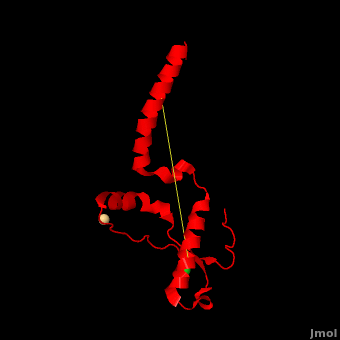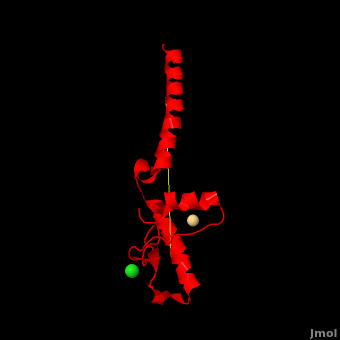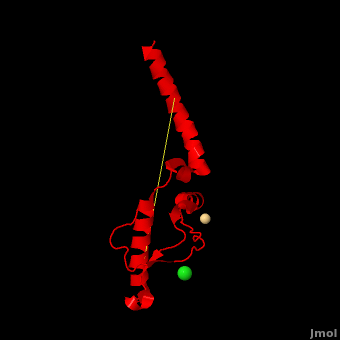
Here we use molecular dynamics simulations to investigate the structural determinants of the globular domain in engineered Mouse (Mo) PrP variants, in WT human (Hu) PrP (PDB: 1hjn) and in WT MoPrP (PDB: 1xyx). The Mo PrP variants investigated here contain one or two residues from Homo sapiens and are denoted “MoPrP chimeras”. (colored in yellow) in in vivo or in in vitro cell-culture experiments, the (in darkmagenta). Our main results are the following: (i) The chimeras resistant to PrPSc infection show between the α1 helix and N-terminal of α3 helix than HuPrP, MoPrP and the non-resistant chimeras (). This is due to stronger specific interactions between these two regions, mainly the and the . (ii) The β2-α2 of PrPC is known to differ in its conformation across different species and is suggested to be responsible for the species barrier of PrPSc propagation. Our simulations detect exchanges between different conformations in this loop which can be categorized into two distinct patterns: some chimeras experience a 310-helix/turn pattern like in MoPrP and others show a bend/turn pattern like in HuPrP. In the Mo-like pattern (colored in green), 310-helix conformation is stabilized by the . In the Hu-like pattern (colored in darkred), a stabilizes the bend conformation. Interestingly, the dominant-negative effect of MoPrP chimeras over WT MoPrP occurs if the chimera not only resists PrPSc infection but also adopts the Mo-like pattern of exchanges between conformations in the β2-α2 loop. This suggests that the compatible loop conformation allows these dominant-negative chimeras to interfere with the conversion of MoPrP to PrPSc.
The structural features presented here indicate that stronger interactions between α1 helix and N-terminal of α3 helix are related to the resistance to PrPC → PrPSc conversion, while the β2-α2 loop conformation may play an important role in the dominant-negative effect.
3D structures of prion
Prion 3D structures