Antifreeze proteins (AFPs) evolved in various organisms permitting their survival in subzero environments[1]. They exhibit remarkable structural diversity and molar activities across the various kingdoms[2]. The longhorn beetle, Rhagium inquisitor, has the ability to supercool to below -25 °C partially due to the presence of a highly potent AFP (RiAFP) in its hemolymph. is a 13-kDa protein with one of the highest antifreeze activities measured for any AFP.
Function
RiAFP, like other AFPs, adsorb to the surface of ice crystals and lower the temperature at which these crystals grow. Consequently, creating a difference between the melting point and the freezing point known as thermal hysteresis (TH), within which the ice growth is arrested[3]. It is well accepted that AFPs inhibit ice crystals growth through the adsorption inhibition model[4]. According to this model AFPs bind to an ice crystal surface and block water molecules from accessing it at the bound location. The ice front thus becomes convex toward the solution between the surface-bound AFPs, creating the microcurvature. Hence, the ice grow is less favorable due to Gibbs-Thompson-Herring (i.e. Kelvin) effect, which leads to noncolligative depression of the freezing point (Tf) of the solution.
Overall Structure
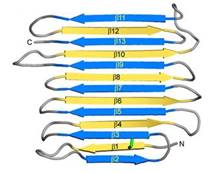
Fig. 1. Secondary structure diagram
The crystallographic structure of
RiAFP was defined recently
[5].
RiAFP has a novel β-solenoid architecture that forms . This sandwich is composed of two parallel remarkably regular . The β-sheets lie on top of each other with the upper and lower strands parallel but in the opposite orientation. β1 and β2 strands are untiparallel to the other strands in each sandwich plane. Two ends deviate from β helix regularity by forming (Figure 1). These capping structures help to prevent end-to-end associations that would impair the solubility of
RiAFP and lead to oligomerization and aggregation.
The three residues in β-strand 11 at the C terminus that are too bulky to be accommodated into the core may also contribute to the capping structure to prevent amyloid-like polymerization. RiAFP solenoid possesses compressed nature with average distance between the sheets of only 6 Å. In the core of RiAFP, the side chains within apposed β-strands from the two β-sheets are staggered, allowing the side chains to interdigitate and pack tightly against one another. Most of the in the core are from Ala, Ser and Thr. Those residues create a more compact fold that may contribute to the high stability and antifreeze activity of RiAFP. Within the core there are between Thr-Ser (65-55, 85-75, 132-124 respectively) and one between Cys4-Cys21, that contribute to stabilization of the whole structure. The β-turns in the structure contain mostly Gly or Pro residues.
The (26-kDa) comprises two RiAFP molecules juxtaposed with their ice-binding surfaces, however the protein is monomer in the solution.
Ice Binding Surface (IBS)
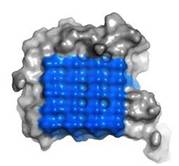
Fig. 2. Ice-binding surface
IBS of RiAFP contains five expanded within the top β–sheet. These motifs are remarkably regular, allowing any rows/columns of TXTXTTX motifs to be exactly superposed onto any other rows/columns. Threonine residuses are crucial for maintaining antifreeze activity. It was found in other AFPs that mutations of the Thrs within these motifs decrease the TH. The Thr hydroxyls define a large flat IBS of 420 Å2, which correlates with high antifreeze activity (Figure 2).
Molecular Basis for Ice Binding
IBS is less hydrophilic than the other β–sheet, which is consistent with its role of interacting with the ice (Figure 3). Adsorption of the AFP ice-binding surface to ice is facilitated by the flatness of the IBS of the RiAFP. The Sc (shape complementarity) values between RiAFP and ice interfaces range from 0.75-0.78, where 1.0 indicates perfect match. For comparison, antigen-antibody complexes usually have their Sc values in the range of 0.64–0.68.
Thr hydroxyls bind 3 ranks of 6 with equivalent spacing between the 4 ranks of Thr side chains. These water molecules are bound tightly, they have lost both translational and rotational freedom and resemble those in an ice lattice. The waters observed in the computational simulation appear to be organized in an ice-like formation, with close matches to the primary prism (Figure 4) and basal planes of ice. IBS is responsible for ordering an ice-like array of anchored “clathrate” water molecules to promote adsorption to ice.
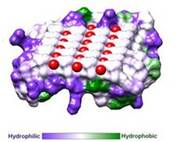 Fig. 3. Hydrophobicity of RiAFP | 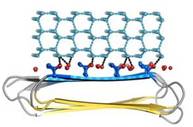 Fig. 4. Docking of RiAFP to the primery prism plane of a hexagonal ice lattice |
An advantage of this indirect binding mechanism is that the organized waters are still fluid enough to make flexible matches to the ice-like quasi-liquid layer around the ice before becoming rigidified as the junction layer freezes. Alignment of IBS of RiAFP to hexagonal ice showed possible interactions of the protein with the several ice planes. Several ranks of matching Thr hydroxyls to water molecules give the RiAFP an ability to bind multiple ice planes, by that providing the protein hyperactivity (Figure 5).
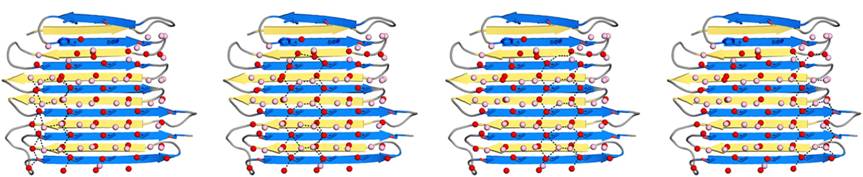
Fig. 5. Four water patterns, that could potentially independently bind the RiAFP to ice.Crystallographic waters are in red; simulation waters are in pink; dashed lines (black) represent potential hydrogen bonds
Quiz





