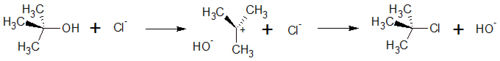
The SN1 reaction belongs to the basic reaction in organic chemistry. The number 1 says that it is a monomolecular reaction. This means that in the rate determining step of the reaction, only one of the educts is involved. The kinetic of the reaction therefore follows the reation rate of first order.
In general, substitutions exchange substituents in an organic molecule. One example of an SN1 reaction is the exchange of the Hydroxide in tert-Butanol by a Chloride Ion or
In general, SN1 substitution can take place when a stable carbocation can be formed. If not, the reaction follows the SN2 mechanism.
The SN1 with the removement of a hydroxide-ion out of the molecule, in this case tert-Butanol. By this, a positively charged carbocation with a planar geometry is formed. This step is also the rate-determing step because it is the slowest step in this reaction.
In the , the haloanion bound to the carbocation, and a neutral haloalkane is formed. With this step, the hydroxy-substituent is replaced by a halogen-substituent.
See also
SN2 reaction: Substitution of chloride and methanol
Esterification
Ozonolysis - a type of cycloaddition
References
This demo was adapted from http://www.chemieunterricht-interaktiv.de/en/animations/sn1_substutition/sn1_substitution_3d.html by Dr. V. Pietzner, part of the ChiLe project

