Student Projects for UMass Chemistry 423 Spring 2012-5
From Proteopedia
Contents |
Caspase 3- 1rhk
Introduction
Caspases are a group of dimeric cysteine proteases that play important roles to control the ultimate steps of apoptosis and innate inflammation; they are also very important in cellular development, homeostasis and in a wide range of diseases such as neurodegeneration, ischemia and cancers. During apoptosis, the initiator caspases (caspases 8 and 9) as upstream regulators cleave and activate with the downstream executioner caspases (caspases 3, 6 and 7). Then the activated executioner caspases will cleave upwards of 500 key proteins and DNA, which finally cause the death of cells.[1]
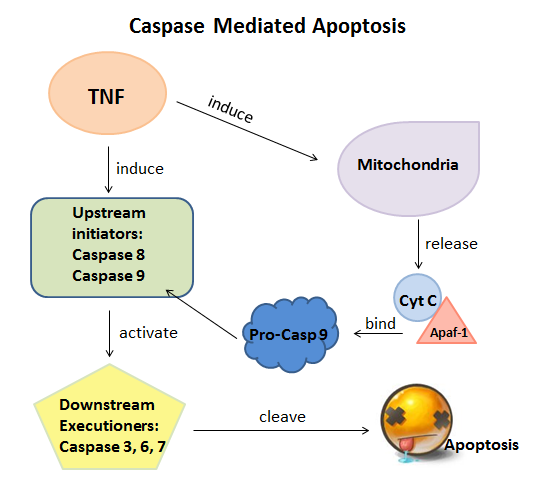
|
There are many caspase structures are researched, and most of them involve either peptide, protein inhibitors or unattractive candidates for drug development.[2] is one of the downstream executioner caspases which interacts with caspase 8 and 9. It is formed from a 32 kDa zymogen that is cleaved into 17 kDa and 12 kDa subunits. It contains four anti-parallel beta sheets from p17 and two from p12, which will come together to make a heterodimer that will interact with another heterodimer to form a total 12-stranded structure surrounded by that is owned by caspases only.[3][4] As an executioner caspase, the caspase-3 zymogen does not have activity until it is cleaved by an initiator caspase after apoptotic signaling events have occurred.[5] Caspase 3 has many of the typical characteristics which all currently-known caspases also own. For instance, its contains a cysteine residue, and histidine residue, . Also, Caspase 3 is able to be activated by diverse death-inducing signals, which includes the chemotherapeutic agents. Pathways of the activation of Caspase 3 was already identified that were either dependent on or independent of mitochondrial cytochrome c release and Caspase 9 function. It also plays an significant role many other apoptotic scenarios in tissue-, cell type-, or death stimulus-specific manner.[6]
Since Caspase 3 cleaves a wide range of cellular substrates including structural proteins and DNA repair enzymes and also activates an endonuclease caspase-activated DNAse, which causes the DNA fragmentation that is characteristic of apoptosis, it has many biological functions, such as normal brain development and several significant diseases including Alzheimer’s disease, Polycystic Kidney Disease and Cancers. In a very frequently-occurring cancer, breast cancer, Caspase 3 acts as a key mediator whose mRNA and protein expression was examined, which leads that the rates of apoptosis as measured by both caspase 3 activation and nucleosome release are higher in breast cancer than in nonmalignant breast tissue.[7] In another cancer, lung cancer, researches shown that polymorphisms in the caspase 3 gene may influence caspse 3 production and activity, thereby modulating the susceptibility to lung cancer.[8] Thus, to cure cancers, studying on Caspase 3 becomes a significant entrance.
Overall Structure
|
Caspase 3, as shown here, is composed of three chains (referred to as A (p17), B(p12), and C) that are 147, 102, and 5 amino acids long (respectively). The three chains are primarily held together through a parallel non-polar beta-strand connection between chains A and B shown , and an anti-parallel polar beta-strand connection between chains B and C shown . Chain A consists of 7 beta-strands and 4 alpha helixes. Chain B has 4 beta-strands and 3 alpha-helices. 6 beta-strands (4 from chain A, and 2 from chain B) form a beta-sheet in the middle of the enzyme surrounded by alpha-helices. This (alpha-helix, beta-strand) causes defining characteristic of caspases and is caused by hydrophobic interactions due to the polarity of the structures shown (hydrophobic, polar).
Only chains A and B are conserved. Chain C consists of a single beta-sheet which can be of variable length and sequence which, though close to the active site on chain A does not seem to be part of it. This is why, though there are three chains shown here, Caspase-3 is considered a heterodimer, and not a heterotrimer. The following example of Caspase-3 with a bromomethoxyphenyl inhibitor does not have a chain C, the inhibitor is considered part of chain A.
The structure shown here is
While the heterodimer shown above and below is the active complex; the original of casepase-3 will form a heterotetramer through an anti-parallel beta sheet interaction with another caspase-3 through each final beta-strand in chain B shown . This forms a containing a non-polar twelve stranded beta sheet surrounded by polar non-polar alpha-helixes is a defining characteristic of caspases, shown (polarity shown , hydrophobic, polar) , particularly caspases-1, 3, 7, and 8.[9] thank you!
Binding Interactions
|
The of the enzyme has been determined to be Cys-285 and His-237. Cys-285 can be seen with its sulfur group and yellow and the unique nitrogen-carbon ring of His-237 is seen in gray and blue. His-237 stablizes the peptide while Cys-285 attacks, cleaving the peptide bond of peptides with sequences Asp-X-X-Asp. In addition to the active site, Cys-285 and Gly 238 stabilize the transition state through hydrogen bonding and therefore also very important. There is a to stabilize the Asp residue of the peptide undergoing cleavage. The pocket,which can be seen as the pink amino acids, consists of polar amino acids such as Arg, Ser, and Gln which allows polar Asp to be held in the correct orientation for the reaction to occur [10] .
Since the catalytic site of the molecule has been determined, inhibitors have been developed which have the ability to bind to these essential amino acids to prevent the enzyme from working. Caspase 3 is regulated with small peptides and other proteins in vivo but since proteins are often large and need specific conditions to work, they are often not ideal to use for medical treatments.
There are numerous inhibitors available commericially as well as research for more specific and easily controlled inhibitors. Ac-DEVD-aldehyde is a commercially available inhibitor of Caspase 3. interacts directly with the Cys 285 of the active site leading to the formation of a hemithioacetal and stablization by hydrogen bonding with Cys 285 and Gly 238 [11]. This inhibits Caspase 3 through competetive inhibition since it binds to the same site as the substrate. All of the colored amino acids in the green scene show the binding sites of the inhibitor with the pink amino acids signifiying the catalytic diyad. This convention will stay throughout all of the inhibitor binding scenes.
Other molecules such as nicotinic acid aldehydes have also been seen to inhibit Caspase 3 and have shown great promise due to peptide-less characteristics. In experimental trials nicotinic acid aldhyde A had a similar as Ac-DEVD-aldehyde binding directly to the catalytic diad and preventing substrate binding. However there are other nicotinic acid aldhydes that have and have shown to have greater inhibition. For example both nicotinic acid aldegydes have strong interactions with Tyr 388 and Phe381 which lead researchers to believe that these amino acids also have an important role in the catalytic mechanism of the enzyme. This reinforces the idea that there are many parts of the protein that are vital for correct function and shape, not exclusively the active sites [12].
After creating a list of for multiple inhibitors, it can been seen that the most important region of binding is around the catalytic diyad. Each of the common amino acids involved in binding it highlighted in a different color and illustrates the importance of the availability of this region for proper function.
Additional Features
|
Activation
Since the function of caspase-3 is so crucial to the death of the cell, having it active all of the time would be highly detrimental to the survival of the cell. As mentioned before, the cell gets around this issue by expressing caspase-3 as an inactive proenzyme called procaspase-3. In the apoptotic pathways, procaspase-3 is activated both by caspase-9 as well as caspase-3 [13]. This is a good example of enzyme regulation; since having more caspase-3 is good for apoptosis activation of procaspase-3 by caspase-3 itself is something very useful for the cell to do.
The most important thing for procaspase-3 to do, however, is block itself from being casually and unintentionally activated. This is accomplished through use of an internal switch of sorts present on the proenzyme that acts as a safety, not unlike a gun. This switch on procaspase-3 is comprised of three Aspartic Acid residues near the intersection of the p17 and p12 subunits [14]. These three residues, Asp 179 – Asp 181, are located near the active site of the enzyme, Cys 163. By removing or modifying this DDD safety, either by an upstream signal or by targeted drugs, procaspase-3 can be converted into caspase-3, thereby activating it.
Unfortunately, the structure for procaspase-3 is not on Proteopedia at all. Luckily for us, caspase-7 undergoes a similar sort of activation (and the structure of both caspase-7 and its proenzyme) are both available. As with caspase-3, the active site of caspase-7 is a cysteine residue, here Cys 186, located on the outside of the molecule. Seen in orange , the presence of this cysteine catalyzes the cleavage reaction that caspase proteins are known for. The residues in black, Arg 187 and Tyr 223, are allosterically stabilized, and the residue in light blue, Gly 188, acts as a hinge of sorts to get the two allosterically stabilized residues in place [15]. If we look at the of caspase-7, we see that the allosterically linked residues are actually rather far apart. This keeps Cys 186 away from the surface of the enzyme and keeps it inactive.
While the activation in procaspase-3 is slightly different, as it involves the removal of as safety catch of sorts, the key idea to take home is that in order for caspases to function, they must be activated. The activation of these caspases leads directly of the exposure of the cysteine that makes up the active site.
Credits
Introduction - Di Lin
Overall Structure - Austin Virtue
Drug Binding Site - Jill Moore
Additional Features - Alex Way
References
- ↑ Jeanne A. Hardy and James A. Wells, Dissecting an Allosteric Switch in Caspase-7 Using Chemical and Mutational Probes, THE JOURNAL OF BIOLOGICAL CHEMISTRY,VOL. 284, NO. 38, pp. 26063–26069, September 18, 2009.http://www.jbc.org/content/284/38/26063.short
- ↑ Crystal structure of the complex of caspase-3 with a phenyl-propyl-ketone inhibitorReducing the peptidyl features of caspase-3 inhibitors: a structural analysis.Becker, J.W., Rotonda, J., Soisson, S.M., Aspiotis, R., Bayly, C., Francoeur, S., Gallant, M., Garcia-Calvo, M., Giroux, A., Grimm, E., Han, Y., McKay, D., Nicholson, D.W., Peterson, E., Renaud, J., Roy, S., Thornberry, N., Zamboni, R.,Journal: (2004) J.Med.Chem. 47: 2466-2474
- ↑ Salvesen GS (January 2002). "Caspases: opening the boxes and interpreting the arrows". Cell Death Differ. 9 (1): 3–5. doi:10.1038/sj.cdd.4400963. PMID 11803369.
- ↑ Lavrik IN, Golks A, Krammer PH (October 2005). "Caspases: pharmacological manipulation of cell death". J. Clin. Invest. 115 (10): 2665–72. doi:10.1172/JCI26252. PMC 1236692. PMID 16200200
- ↑ Walters J, Pop C, Scott FL, et al. (December 2009). "A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis". Biochem. J. 424 (3): 335–45. doi:10.1042/BJ20090825. PMC 2805924. PMID 19788411
- ↑ Emerging roles of caspase-3 in apoptosis,Alan G Portera and Reiner U Jänicke,Institute of Molecular and Cell Biology, The National University of Singapore, 30 Medical Drive, Singapore 117609, Republic of Singapore: http://www.nature.com/cdd/journal/v6/n2/abs/4400476a.html
- ↑ Caspase 3 in breast cancer.,O'Donovan N, Crown J, Stunell H, Hill AD, McDermott E, O'Higgins N, Duffy MJ. Source, Department of Medical Oncology, St. Vincent's University Hospital, University College Dublin, Dublin 4, Ireland. http://www.ncbi.nlm.nih.gov/pubmed/12576443
- ↑ Identification of Polymorphisms in the Caspase-3 Gene and Their Association With Lung Cancer Risk Jin Sung Jang,Kyung Mee Kim,Jin Eun Choi,Sung Ick Cha,Chang Ho Kim,Won Kee Lee,Sin Kam,Tae Hoon Jung and Jae Yong Park, Department of Biochemistry, School of Medicine, Kyungpook National University, Daegu, Korea. http://onlinelibrary.wiley.com/doi/10.1002/mc.20397/pdf
- ↑ Salvesen GS "Caspases: opening the boxes and interpreting the arrows" Nature Publishing Group 2002 vol:9 iss:1 pg:3 -5
- ↑ Cohen G. M. (1997). Caspases: the executioners of apoptosis. Biochem. J. 326(Pt 1), 1–16.short
- ↑ Becker JW, Rotonda J, Soisson SM, Aspiotis R, Bayly C, Francoeur S, Gallant M, Garcia-Calvo M, Giroux A, Grimm E, Han Y, McKay D, Nicholson DW, Peterson E, Renaud J, Roy S, Thornberry N, Zamboni R. Reducing the peptidyl features of caspase-3 inhibitors: a structural analysis. J Med Chem. 2004;47:2466–2474.short
- ↑ Becker JW, Rotonda J, Soisson SM, Aspiotis R, Bayly C, Francoeur S, Gallant M, Garcia-Calvo M, Giroux A, Grimm E, Han Y, McKay D, Nicholson DW, Peterson E, Renaud J, Roy S, Thornberry N, Zamboni R. Reducing the peptidyl features of caspase-3 inhibitors: a structural analysis. J Med Chem. 2004;47:2466–2474.short
- ↑ Fan, Ting-Jun, Han, Li-Hui, Cong, Ri-Shan, and Liang, Jin. “Caspase Family Proteases and Apoptosis”. Acta Biochimica et Biophysica Sinica. Vol. 37 No. 11 (2005): 719 – 27. Web. http://www.abbs.info/pdf/37-11/1-719-727-05165.pdf
- ↑ Porter, Alan G. “Flipping the safety catch of procaspase-3”. Nature Chemical Biology. Vol. 2. No. 10 (2006): 509 – 10. Web. http://www.nature.com/nchembio/journal/v2/n10/full/nchembio1006-509.html
- ↑ Hardy, Jeanne A. and Wells, James A. “Dissecting an Allosteric Switch in Caspase-7 Using Chemical and Mutational Probes” The Journal of Biological Chemistry. Vol. 284. No. 38 (2009): 26063 – 69. Web. http://www.jbc.org/content/284/38/26063.full