Function
TGF-β receptors (Transforming Growth Factor) (TGFR) are serine/threonine kinase receptors. They are involved in paracrine signaling and are found in many types of tissue. TGF-β ligands include bone morphogenetic proteins, growth and initiation factors, anti-Mullerian hormone, activin, nodal TGF-β[1]. There are 3 types of TGFR:
- TGFR I forms heteromeric complex with TGFR II when it is bound to TGF-β. The complex transduces the TGF-β signal from the cell surface to the cytoplasm by phosphorylating proteins which regulate the transcription of genes related to cell proliferation. TGFR I has high affinity for TGF-β1 and low affinity for TGF-β2.
- TGFR II is a tumor suppressor transmembrane protein. TGFR II has high affinity for TGF-β1 and low affinity for TGF-β2.
- TGFR III is a cell-surface chondroitin sulfate / heparin sulfate proteoglycan. It acts as a reservoir of ligand for TGFRs. TGFR III has high affinity for TGF-β1, TGF-β2 and TGF-β1.2.
See also Receptor, TGF beta signaling pathway, and Growth factors.
Disease
Over-expression of TGF causes kidney disease, diabetes and renal disease. Mutations in TGFR II cause various types of tumors[2].
Structural highlights
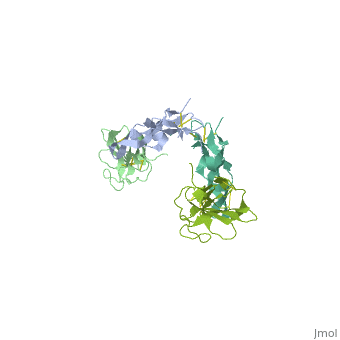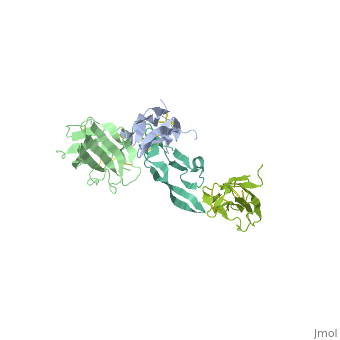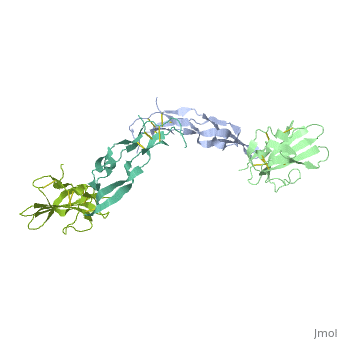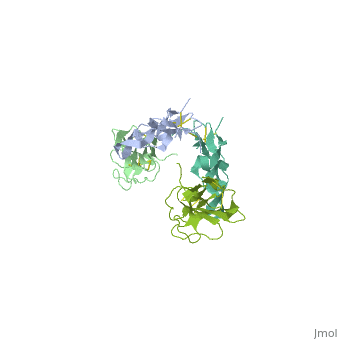
TGFR structure contains a 100-140 residues ligand-binding N-terminal extracellular domain; a transmembrane domain; a 350-400 amino acid cytoplasmic kinase domain; and a C-terminal zona pellucida (ZP) domain of ca 260 residues which has a role in protein polymerization.
3D Structures of TGF-beta receptor
TGF-beta receptor 3D structures