Function
Tumor necrosis factor receptor (TNFR) or death receptor is a trimeric cytokine receptor which binds TNF[1]. TNFR family contains several members and superfamily (TNFRSF) members.
- TNFRSF 1 is called Lymphotoxin-α or TNF-β[2];
- TNFRSF 3 is called TNFR-III;
- TNFRSF 4 is called OX40L receptor;
- TNFRSF 5 is called CD40L receptor;
- TNFRSF 6 is called Fas;
- TNFRSF 9 is called 4-1BBL;
- TNFRSF 10 see TRAIL;
- TNFRSF 10B is called Dr5;
- TNFRSF 11A is called RANK;
- TNFRSF 11B is called Osteoprotegerin;
- TNFRSF 12 is called TWEAK;
- TNFRSF 12A is called TWEAKR;
- TNFRSF 13B is called BAFF or sTALL-1;
- TNFRSF 13C is called BAFF receptor;
- TNFRSF 14 is called LIGHT;
- TNFRSF 16 is called Low-affinity nerve growth factor receptor; p75NTR; p75 neurotrophin receptor;
- TNFRSF 18 is called GITRL;
- TNFRSF 21 is called Dr6;
- TNFRSF 25 is called Dr3;
See also
Relevance
TRAPS - a condition characterized by recurrent episodes of fever is associated with TNFR[3].
Structural highlights
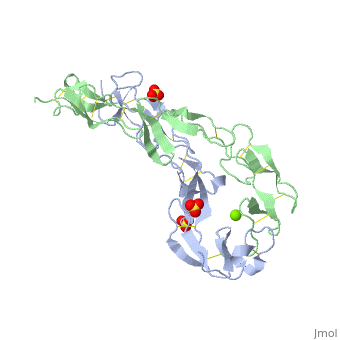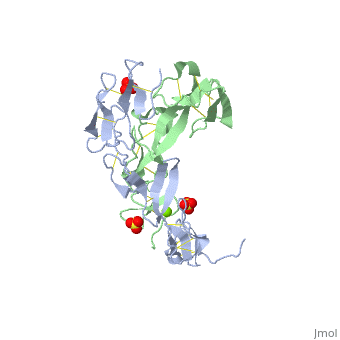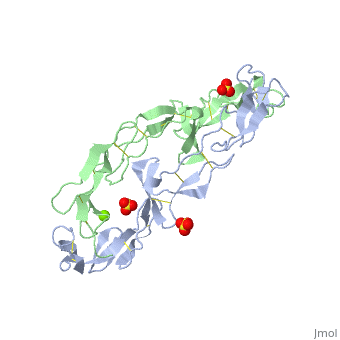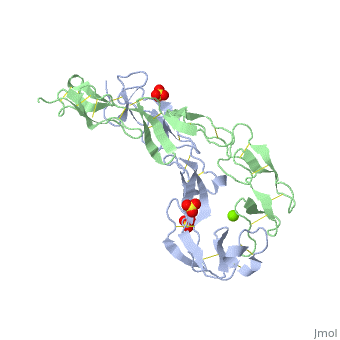
The extracellular domain of TNFR contains 2 to 6 cysteine-rich domains (CRD). The . The CRDs are involved in binding of TNF[4]. . Water molecules are shown as red spheres.
3D structures of tumor necrosis factor receptor
Tumor necrosis factor receptor 3D structures