User:Mary Ball/AFP
From Proteopedia
| |||||||||
| 1wfb, resolution 1.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Standard Residues: | |||||||||
| Related: | 1wfa | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
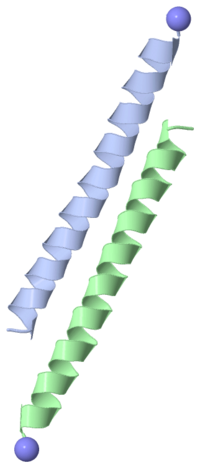
WINTER FLOUNDER ANTIFREEZE PROTEIN
Type I antifreeze proteins (AFPs) are grouped together according to similar structure. The one shown on the right is from the winter flounder. This AFP consists of a chain of 37 amino acids: DTASDAAAAAALTAANAKAAAELTAANAAAAAAATAR The chain forms a single alpha-helix.
The four threonines are evenly spaced in the chain, such that there are three 11-amino-acid sequences. In the single alpha-helix that forms, the threonines all lie on one "face" of the helix.
Baardsnes, et al (1999) created mutations that reduced the protein's ice-binding ability. They also compared the sequences of five different type I AFP molecules. They concluded that the "face" with the repeated Threonines promotes binding to ice crystals.
REFERENCE
New ice-binding face for type I antifreeze protein., Baardsnes J, Kondejewski LH, Hodges RS, Chao H, Kay C, Davies PL, FEBS Lett. 1999 Dec 10;463(1-2):87-91. PMID:10601644
ABOUT THIS STRUCTURE
1WFB is a 2 chains structure with sequences from Pseudopleuronectes americanus. The December 2009 RCSB PDB Molecule of the Month feature on Antifreeze Proteins by David Goodsell is 10.2210/rcsb_pdb/mom_2009_12. Full crystallographic information is available from OCA.
REFERENCE
- Sicheri F, Yang DS. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995 Jun 1;375(6530):427-31. PMID:7760940 doi:http://dx.doi.org/10.1038/375427a0
Page seeded by OCA on Thu Jan 21 08:56:47 2010
WINTER FLOUNDER ANTIFREEZE PROTEIN ISOFORM HPLC6 AT-180 DEGREES C
Antifreeze proteins provide fish with protection against the freezing effect of polar environments by binding to ice surfaces and inhibiting growth of ice crystals. We present the X-ray crystal structure at 1.5 A resolution of a lone alpha-helical antifreeze protein from winter flounder, which provides a detailed look at its ice-binding features. These consist of four repeated ice-binding motifs, the side chains of which are inherently rigid or restrained by pair-wise side-chain interactions to form a flat binding surface. Elaborate amino- and carboxy-terminal cap structures are also present, which explain the protein's rich alpha-helical content in solution. We propose an ice-binding model that accounts for the binding specificity of the antifreeze protein along the <0112> axes of the (2021) ice planes.
Ice-binding structure and mechanism of an antifreeze protein from winter flounder., Sicheri F, Yang DS, Nature. 1995 Jun 1;375(6530):427-31. PMID:7760940
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.