Vibrio cholerae colonization factor TcpF
From Proteopedia
IntroductionMolecule: Toxin coregulated pilus biosynthesis protein F Type:protein Length: 318 Organism:Vibrio Cholerae TcpF is a toxin-coregulated pilus that facilitates colonization of vibrio cholerae in the intestine. Vibrio cholerae relies on two main virulence factors: toxin-coregulated pilus (TCP) and cholera toxin (CT) to cause the gastrointestinal disease cholera. TCP, encoded by the tcp operon, is a type IV pilus that mediates bacterial autoagglutination and colonization of the intestine.[1] Structure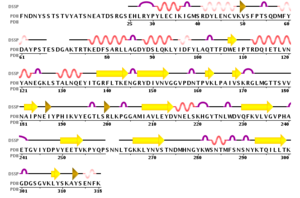 Secondary Structure of TcpF, generated by PDB 1xtc  TcpF-RNA 1xtc The structure of TcpF is consisted with an and a connected by an extended . In detail, the NTD is composed of a short twisted β-sheet encapsulated by seven short α-helices with a second twisted β-sheet forming the floor of this domain. The NTD is connected with The CTD, which consists of two twisted antiparallel β-sheets.[1] TCP is a filamentous structure belongs. Studies showed that the regions, close to CTD are important for mediating colonization. The architecture of TcpF, with discrete NTD and CTD joined by a linker, is flexible that allow to accommodate a larger substrate. FunctionThe function of many of Tcp genes and their associated proteins are largely unknown. TcpF is the only protein secreted by the TCP apparatus and it represents the first nonpilus protein identified that is specifically secreted outside the bacterial cell by a type IV pilus biogenesis apparatus.[1] Studies hypothesize that TcpF, identified in classical isolates of V. cholerae O1 is an essential factor for colonization in the infant mouse cholera model. Bacteria lacking tcpF are deficient in colonization, and anti-TcpF antibodies are protective in the infant mouse cholera model. TcpF is expressed in vivo during human infection and generates a substantial immune response in patients infected with V. cholera. By looking at the homology of TcpF, the NTD and the CTD together form a unique interface that interacts with partner proteins to function in V. cholerae colonization.[2] Evolution Multiple Sequence Alignment, generated by Biology Workbench 1xtc 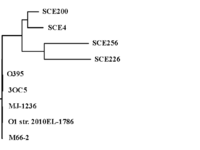 Phylogenetic tree-Tcp, generated by Biology Workbench 1xtc The environmental TcpF proteins are important for secretion because they retained a high degree of identity in the region. Each of the environmental TcpF proteins examined was secreted from the pathogenic strains. Now, TcpF genes from six pathogenic strains of V. cholerae have been sequenced, and all of these strains have nearly identical TcpF amino acid sequences.However, studies examined that environmental TcpF proteins cannot mediate colonization in the infant mouse cholera model. By comparing the environmental strain, SCE4 and the pathogenic TcpF, O395 (See Multiple sequence alignment result ), it revealed that the regions, divergent from one another are located at the middle of the C terminal domain. Therefore, lack of colonization mediated by environmental TcpF proteins may suggest that the divergent regions between the pathogenic and environmental proteins are essential for TcpF function. [1] ApplicationV.cholerae has a variety of proteins in the periplasm, but only a small part are known to be transported across the outer membrane into the extracellular space. The secretion systems (TCP system) is able to recognize their cognate substrates for export. The TCP apparatus is required by the secretion of TcpF. TcpF is absolutely necessary for colonization and V.cholerae pathogenesis. However, The mechanism for the action of TcpF remains to be elucidated.[3] 3D structures of colonization factor antigenSee Colonization factor antigen References
| ||||||||||||
