Introduction
is a viral encoded enzyme that converts the viral single-stranded RNA genome into a double-stranded DNA provirus that is integrated into the host chromosome in the host cell's nucleus. The process of converting viral ssRNA into dsDNA that can incorporate into the host chromosome is called retrotranscription, and is characteristic of all retroviruses. HIV-1 reverse transcriptase is encoded by the human immunodeficiency virus, well known as the etiological agent of acquired immune deficiency syndrome (AIDS).
RT performs three catalytic steps: 1) RNA-dependent DNA polymerization to create a negative sense DNA strand that complements the positive sense viral RNA genome, 2) ribonuclease H cleavage of RNA in the RNA:DNA heteroduplex, and 3) DNA-dependent DNA polymerization to make a dsDNA using the previously synthesized negative sense DNA strand as a template. The dsDNA is transported to the nucleus where it integrates into the host cell's chromosome. HIV-1 is chronic and requires lifelong treatment with a combination of at least three different antiviral drugs. In addition, the emergence of drug-resistant HIV-1 strains means drugs with new viral targets are constantly being developed. See also Reverse transcriptase.
Structure of RT domains
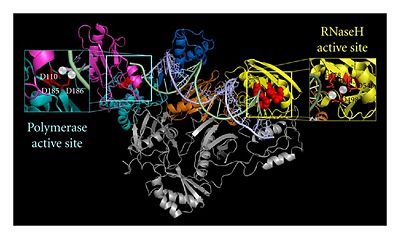
RT Polymerase and RNase H domains. Reprinted from Esposito et al PMID:22778958 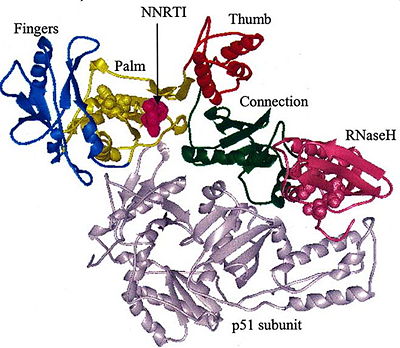
The subdomains of RT. Reprinted from Sluis-Cremer et al PMID:15544453 RT is an composed of a 560 amino acid 66kDa subunit (p66) and a 440 amino acid 51kDa subunit (p51). The p66 and p55 domains are derived from cleavage of the same polyprotein precursor. The p51 is made from the C-terminal cleavage of the p66 subunit by HIV-1 protease. As a result, they share a common amino terminus, but the p51 subunit does not have an RNase H domain.
The p66 subunit contains two enzymatically active domains, polymerase and RNase H. This polymerase is responsible for catalyzing the polymerization of DNA using either RNA or DNA as the template. There are three asp residues (D110, D185, D186) in polymerase's active site that play a key role in the enzyme's function and are referred to as the .[1] The endonucleolytic ribonuclease H (RNase H) specifically degrades the RNA strand of RNA:DNA duplexes that are produced during retrotranscription. RT has a right-hand structure.[2] The polymerase domain can be divided into several subdomains: the fingers (residues 1-85 and 118-155), palm (residues 86-117 and 156-236), thumb (237-318) and connecting (319-426). The RNase H domain consists of the C-terminal residues 427-560.[3]
The p51 subunit contains the same four subdomains as the polymerase domain in p66, but in different positions. The p51 subunit is therefore non-enzymatic, and instead stabilizes the proper folding of the catalytic p66 subunit. Instead of adopting an "open" catalytically-active conformation that can accommodate a nucleic acid template strand like p66, the p51 subunit is in a "closed" conformation and plays a largely structural role.[4]
NNRTIs: Anti-retroviral Drugs
RT is a prime target for anti-HIV drugs because of its essential role in the viral life cycle. A wide variety of drugs have been developed to target this enzyme in order to decrease the infectivity of HIV and slow the progression of this chronic disease. Of the 26 anti-retroviral drugs approved by the FDA to treat individuals infected with HIV, 13 target the viral polymerase of RT.[5] One class of drugs, called non-nucleoside reverse transcriptase inhibitors (NNRTIs), contain compounds that bind noncompetitively to a hydrophobic pocket near the polymerase active site. NNRTIs are a group of small hydrophobic compounds with diverse structures that allosterically inhibit HIV-1 but not HIV-2 RT.[3] Recent pre-steady kinetics studies suggest that the conformational state and not the chemical step leading to nucleotide incorporation is blocked by a NNRTI, favoring the "primer grip distortion" model.[6] Binding of these compounds allosterically inhibit RT, causing its distortion and incompetent binding to dNTP.
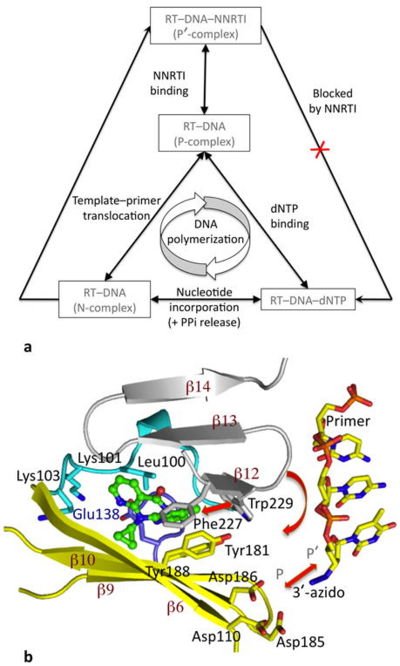
Effect of NNRTI binding on DNA polymerization by RT. Reprinted by permission from Macmillan Publishers Ltd: Nature Structural & Molecular Biology, copyright 2012 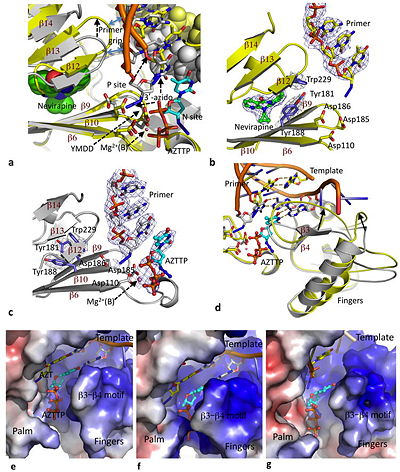
Effects of nevirapine on pol active site conformation and dNTP binding. Reprinted by permission from Macmillan Publishers Ltd: Nature Structural & Molecular Biology, copyright 2012 The NNRTI Binding Pocket
Although nonnucleoside RT inhibitors are structurally diverse compounds, they all bind RT in the same location - . The pocket is located in the palm domain of the p66 subunit between the β6-β10-β9 and β12-β13-β14 sheets approximately 10 angstroms from the three catalytic asp residues that make up the polymerase active site.[4] The NNRTI BP is mostly hydrophobic in nature with considerable aromatic residues (Y181, Y188, F227, W229, and Y232), but also contains several hydrophilic residues (K101, K103, S105, D192, and E224 of the p66 subunit and E138 of the β7-β8 loop of the p51 subunit).[3] NNRTIs most likely access the binding pocket at the p66/p51 heterodimer interface surrounded by .[7] These residues are colored tan in the binding pocket scene. Actually, in the absence of ligand, the side chains of point into the core, so the binding pocket doesn't exist in the free enzyme. The binding of NNRTI to HIV RT causes these side chains to shift away and make room for the ligand to enter the binding pocket.[7]
Effects of nevirapine binding on the RT-DNA complex
Nevirapine is a first generation NNRTI, which binds RT in a butterfly-like conformation.[8] When DNA binds RT, the polymerase is stabilized by the presence of incoming dNTPs and is said to be in a polymerase-competent state. This is the only way the DNA-RT complex can bind dNTP and incorporate nucleotide. However, nevirapine has a destabilizing effect resulting from decreased interaction of key regions of the polymerase active site with nucleic acid. As previously mentioned, nevirapine leads to opening of the NNRTI binding pocket as a result of switching of the Tyr181 and Tyr188 rotamer conformations and shearing of the β12-β13-β14 sheet away from the β6-β10-β9 sheet.[9] The β6-β10-β9 sheet contains the polymerase "catalytic triad" and the β12-β13-β14 sheet contains the "primer grip" that positions the primer strand for polymerization. Nevirapine binding causes the primer grip to shift by 4 angstroms, lifting the terminus of the DNA primer away from the P site, and diminishing interactions of the primer terminus with the conserved catalytic Y183MDD motif.[9] Therefore, the first effect of nevirapine is loosening of the "primer grip."
The second significant conformational change wrought by nevirapine is on the finger subdomain. The nevirapine-tertiary structure deforms the β3-β4 motif, which usually base pairs with the first template overhang, and interacts with incoming dNTPs that will be incorporated during polymerization. As a result of changes to the β3-β4 motif, parts of the fingers are shifted by 5-7 angstroms into regions that would normally accommodate the template overhang in catalytically-competent RT-DNA molecules.[9] This puts the finger subdomain in an open or semi-open conformation.
Third, the nevirapine-ternary structure extends the conformation of the thumb subdomain making it rigid. This is due to restricted inward movement of the thumb by the displaced β12-β13-β14 sheet.[4]
The crystal structure of nevirapine in solution with RT and DNA showed that the primer grip distortions locked the thumb in a hyper-extended position, resulting in diminished interactions between DNA and the polymerase domain. The flexible fingers, altered template-primer, and shifted template-overhang all reduce the productive binding of dNTPs. It appears that the flexibility of the fingers allow dNTP to still bind the RT-DNA-nevirapine complex at the polymerase active site, however, in a polymerase incompetent mode. The exact mechanism of how dNTPs are able to bind in such a nonproductive manner is yet to be uncovered. The dNTPs may enter the N site and bind in multiple orientations, or they may bind in an ordered yet non-productive fashion once the nucleic acid duplex has slid past the polymerase active site.[10]
HIV-1 RT resistance mutations
Binding of DNA to the polymerase active site requires the primer grip to be near the active site in order for the interaction to be catalytically competent. Binding of nevirapine interferes with the primer grip, moving it away from the active site. A bound nonnucleoside inhibitor is surrounded on three sides: 1) β6-β10-β9 sheet, 2) β12-β13-β14 sheet, and 3) 100-105 loop of p66 + Glu138 loop of p51.[9] Chemical changes in these motifs could lead to the facilitated entrance and exit of most nonnucleosides. In many cases, single amino acid mutations in the binding pocket can significantly decrease the antiviral potency of nevirapine. The mutation K103N confers resistance to a wide variety of nonnucleosides. The Y181C and Y188C mutations also confer drug resistance because they essentially control nonnucleoside movement into and out of the binding pocket depending on the orientations of their side chains. In addition, loop mutations (E138K and K103N) can facilitate exit of nonnucleosides from the binding pocket.[9] To combat this problem, researchers have developed second generation NNRTIs that are generally more effective against a range of drug resistant strains.
Conclusion
Analysis of the crystal structures of RT-DNA complexes in the presence and absence of nevirapine allows us to determine the effects of nonnucleosides on RT structure and function. Binding of nevirapine reveals several changes to the RT-DNA complex. It directly displaces the primer grip located in the palm subdomain, which causes the primer terminus to relocate, the thumb to lock in hyper-extension, decreased interaction between DNA and the polymerase domain, and distortion the dNTP binding site. As a result of the conformational changes nevirapine causes, the process of DNA synthesis by RT is essentially inhibited. Unfortunately, the high mutation rate caused by HIV-1 polymerase's low fidelity readily produces mutant strains that are resistant to NNRTIs. Understanding the precise mechanism of these compounds' inhibition is a key step in developing more effective drugs against HIV.