2Fe–2S clusters
ISC-like [2Fe-2S] ferredoxin (FdxB) dimer from Pseudomonas putida JCM 20004: Structural and electron nuclear double resonance characterization[1]
The has a βαββαβ fold with the β-grasp/ubiquitin-like fold motif as found in regular eukaryal and bacterial [2Fe-2S] ferredoxins (e.g. 1i7h, 1cje, 1e9m). FdxB is folded into an (α+β) . In the lattice displaying the . Each protomer binds a that is , where the and the . In the , suggesting that a rapid interprotomer electron transfer between them would be unlikely to occur. In the place of the consensus free cysteine usually present near the [2Fe-2S] cluster of ISC-like ferredoxins, FdxB has the Oδ2. Thus, the overall FdxB structural features argue for its primarily electron transfer role in the cognate ISC system, rather than the direct catalytic function.
The site of reduced iron in the reduced FdxB is the outermost Fe1 site with the low negative spin density, while the innermost Fe2 site with the high positive spin population is the non-reducible iron retaining the Fe3+-valence of a reduced cluster. From a structural point of view, the larger number of polarized (or polarizable) bonds (NH, OH) and the . These results suggest a significant distortion of the electronic structure of the reduced [2Fe-2S] cluster under the influence of the protein environment around each iron site in general.
Rieske Fe-S protein
Cytochrome bc1 (Cbc1) functions as the central pump which transfers protons across the cell membrane. The protons are used to power the rotation of ATP synthase. Cbc1 binds ubiquinol which carries hydrogen atoms. Cbc1 separates the protons and the electrons. The protons are released in the inner side of the membrane for use by ATP synthase and the electrons are transferred to cytochrome c or to the outer side of the membrane. Plants use cytochrome b6f in the same manner binding plastoquinol as a hydrogen carrier. Stigmatellin inhibits the Cbc1 electron transfer by binding to its quinone oxidation site. Antimycin inhibits Cbc1 by binding to its quinone reduction site.[2]
More details in Complex_III_of_Electron_Transport_Chain.
Structural highlights
Cbc1 is a composed of 11 proteins and cofactors which include heme-carrying proteins like and and iron-sulfur cluster proteins like . The iron containing moieties are , (where vinyl side chain of heme are replaced by thioether) and . [3]
4Fe–4S clusters
Structure of adenine DNA glycosylase containing Fe4S4 cluster complex
Adenine DNA glycosylase is also called MutY. MutY has a role in prevention of DNA mutations resulting from oxidative damage forming the mutated base oxoG. DNA polymerase misreads oxoG and pairs it with adenine instead of cytosine. MutY removes adenine from the mismatched oxoG:A pair. MutY contains a 4Fe4S cluster. MutY uses base flipping to twist the mispaired adenine out of the DNA helix and into the MutY active site. MutY contains 2 domains: C-terminal domain and catalytic domain containing a helix-hairpin-helix.
- .
- .
- .
- (PDB code 1rrs)[4]. Water molecules shown as red spheres.
4-hydroxy-2-methylbut-2-enyl diphosphate reductase
4-hydroxy-2-methylbut-2-enyl diphosphate reductase (IspH or HMBPP reductase) is an containing protein. IspH converts 1-hydroxy-2-methylbut-2-enyl 4-diphosphate into isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). IspH participates in isoprenoid biosynthesis. IspH is the last enzyme in the nonmevalonate pathway. [5] IspH is involved in penicillin tolerance.
- is shown.
- .
- Of note, .
- .
- . Water molecules are shown as red spheres.
- (PDB code 3szl).[6]
Acetyl-CoA synthase IV subunit α from Carboxydothermus hydrogenoformans
in Acetyl-CoA synthase IV subunit α from Carboxydothermus hydrogenoformans (1ru3).[7] Water molecules shown as red spheres.
N-Butylisocyanide Oxidation at the [NiFe4S4OHx]-cluster of CO Dehydrogenase[8]
Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO2 as their sole carbon and/or energy source. The with ~ 130 kDa containing , called B, B’, C, C’ and D. Each subunit contains the and cubane-type [Fe4S4] . Another [Fe4S4] connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe4S4OHx] (CO + H2O ↔ CO2 + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H2 and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster.
The high resolution crystal structure of CODH-II from Carboxydothermus hydrogenoformans (dmin = 1.28 Å) revealed (the product of n-BIC oxidation), in which the (2yiv) rather than the (3b52) and states (3i39). , while . A superposition of the CODH-II structures with different ligands bound to the C-cluster reveals a flexible coordination and geometry for the Ni-Fe1 dyad, while the [Fe3S4] moiety of C-cluster remains unchanged in position.
, which was shown to be a binding place for Xe atoms in CODH/ACS (2z8y). Analysis of the CODH-II structure identified the presence of two different channels (see below), in which one is analogous to the channel identified by Xe-soaking in bifunctional CODH/ACS (conserved channel), while the other seems to be specific for monofunctional CODH (unique channel). The conserved channel in CODH-II is similar in position to that in bifunctional CODH, where the channel is extended to reach the A-cluster of ACS. Monofunctional CODHs have smaller side chains like Thr and Val, while Tyr617 and Phe634 block a passage of the unique channel to the protein surface in bifunctional CODH/ACS. Furthermore, the unique channel is completely blocked by residue His9 – Pro13 to prevent diffusion of gaseous substrate/product from the protein in bifunctional CODH/ACS. However, the channel of monofunctional CODH-II is directed towards the solvent, which is in line with its role to allow fast progress and egress of substrates and products from the active site to the outside of the enzyme, which has not been described previously.
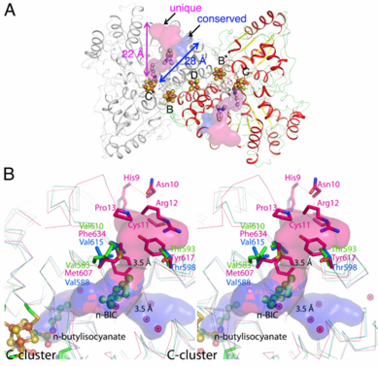
(A) Channels in monofunctional CODH-II
Ch. The channel into which the alkyl group of n-BIC is directed, shown in
pink color, is unique to monofunctional CODHs.
Blue space-filling model shows the channel found in both mono- and bi-functional CODHs. (B) Comparison of channels in mono- (CODH-II
Ch in
green from this study and CODH from
Rhodospirillum rubrum in
cyan (
1jqk)) and bi-functional (CODH from
Moorella thermoacetica in
pink from PDB-ID
2z8y) CODHs.
Pink-colored dots are Xenon atoms found in CODH from
Moorella thermoacetica.
3Fe–4S clusters
Crystal structures of the all cysteinyl coordinated D14C variant of Pyrococcus furiosus ferredoxin: [4Fe-4S] <-> [3Fe-4S] cluster conversion[9]
Structures of the all cysteinyl coordinated D14C variant of ferredoxin from the hyperthermophilic archaeon Pyrococcus furiosus have been determined for the <-> and forms (). The [4Fe-4S] form diffracted to 1.7 Å and two different types of molecules were found in the crystal (2z8q). They have different crystal packing and intramolecular disulfide bond conformation. The crystal packing reveals a (shown in blue and green) in adjacent asymmetric units, while (shown in red and yellow). The while the intramolecular disulfide bond in the (, molecule A is shown in blue and molecule B in green).
![pH dependent equilibrium of D14C [3Fe-4S] P. furiosus ferredoxin between protonated and deprotonated monomers and formation of a disulfide bonded dimer from deprotonated monomers. Fd is short for ferredoxin.](/wiki/images/thumb/0/0b/Schem1.png/300px-Schem1.png)
pH dependent equilibrium of D14C [3Fe-4S]
P. furiosus ferredoxin between protonated and deprotonated monomers and formation of a disulfide bonded dimer from deprotonated monomers. Fd is short for ferredoxin.
Two forms of D14C [3Fe-4S] Pyrococcus furiosus ferredoxin are obtained when purified at pH 8.0: a monomer and a dimer connected by an intermolecular disulfide bond (see static image at the left). When purified at pH 5.8, only the monomer is obtained. The [3Fe-4S] form diffracted to 2.8 Å resolution and showed only the . Crystal packing in (shown in red and orange), which is the same as (1sj1, shown in blue and cyan) even though the space groups are different (see also corresponding side views for ) and ).
Heterometallic [AgFe3S4] ferredoxin variants – synthesis, characterization and the first crystal structure of an engineered heterometallic iron-sulfur protein[10]
The crystal structure of the Pyrococcus furiosus (Pf) ferredoxin (Fd) D14C variant with the novel [AgFe3S4] heterometallic cluster was determined to 1.95 Å resolution (PBD entry 4dhv), being the first reported structure of an engineered heterometallic iron-sulfur protein.
The crystal structure of the shows that the (clearly seen on the electron density map), as predicted from previous spectroscopic and electrochemical studies. The heterometal is coordinated to the and to the (residues are coordinated with Fe ions of heterometal), [Fe4S4] D14C variant (2z8q, heterometallic [AgFe3S4] protein is in cyan and homometallic [Fe4S4] is in green) and completing the incomplete cuboidal cluster present in the [Fe3S4] WT Pf Fd (PDB: 1sj1) and its D14C (PDB: 3pni) variant (for more details see also "Crystal structures of the all cysteinyl coordinated D14C variant of Pyrococcus furiosus ferredoxin: (4Fe-4S) <-> (3Fe-4S) cluster conversion"). Structure alignment of backbone atoms from the heterometallic [AgFe3S4] protein and the homometallic [Fe4S4] D14C variant (2z8q) shows , i.e. the root mean square deviation (RMSD) is 0.4 – 0.7 Å, observed due to the alternate conformation of the main chain atoms, flexible loops and small changes at the N- and C-termini (heterometallic [AgFe3S4] protein is in cyan and homometallic [Fe4S4] is in green). can be seen in the superimposed Fe–S clusters (atom colors corresponding to yellow: S, orange: Fe, gray: Ag) from these two variants, which is due to the presence of the second row transition metal ion (Ag) coordinated to the four S-ligands, i.e. the presence of Ag results in a distorted geometry of the cluster compared to the all-iron arrangement, Ag – S bond lengths compared to Fe – S bonds. However, the S – Ag – S are still close to the expected 90° for a primitive cubic system.

![pH dependent equilibrium of D14C [3Fe-4S] P. furiosus ferredoxin between protonated and deprotonated monomers and formation of a disulfide bonded dimer from deprotonated monomers. Fd is short for ferredoxin.](/wiki/images/thumb/0/0b/Schem1.png/300px-Schem1.png)
