Function
Finasteride, branded as Proscar or Propecia, is a synthetic 4-azasteroid compound that acts as a 5α-reductase inhibitor.[1] The 5α-reductase enzyme is very important in the metabolism of many of the steroids produced by the body, in particular the conversion of testosterone to dihydrotestosterone (DHT).[1] For this reason, Finasteride is used as a treatment for benign prostate hyperplasia (BPH), which is caused by an overproduction of DHT in the male prostate.[2] Androgenetic Alopecia or male pattern baldness (MPB) is another condition in men caused by the build up of DHT, which can also be treated with Finasteride.[3] Finasteride's affinity for 5α-reductase approaches that of testosterone, and can bind to two of the three isoenzymes of 5α-reductase, types I and II.[2]
Structure
To see what Finasteride looks like unbound, click this link: . Finasteride belongs to the subclass of steroids called azasteroids. All steroids contain a core structure called a gonane, a structure composed of 3 cyclohexane rings and 1 cyclopentane ring "fused" together.[4] Although a gonane has 6 chirality centers, giving it 64 possible steroisomers, the majority of steroids contain the 5α-gonane stereoisomer. [5] The function and naming of steroids are determined by what functional groups are attached to the rings of gonane and by the substitution of carbon atoms in the rings with different atoms. In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids.[4] The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide with a tert-butyl group bonded to the nitrogen atom, has been added onto the carbon 17 (Figure 1).
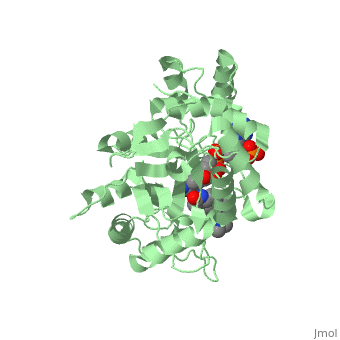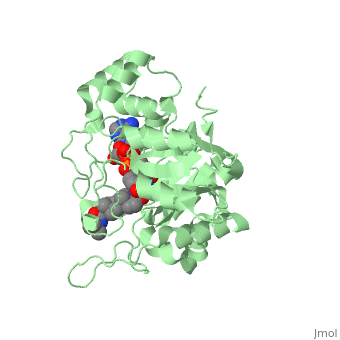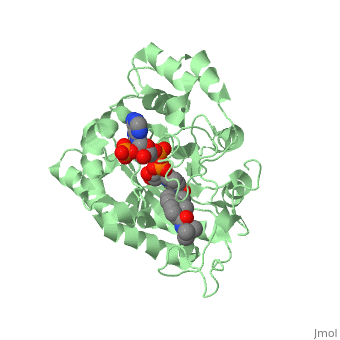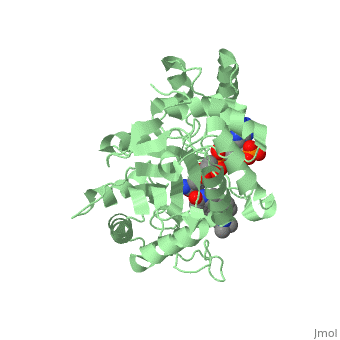
There is no known three-dimensional structure of 5α-redcutase to show how it binds with Finasteride. However, there is a known structure for the enzyme 5β-reductase bound to Finasteride and NADP by a mechanism that is similar to the binding of 5α-reductase to Finasteride. The interactions between 5β-reductase and Finasteride and NADP were determined by using the homology modeling software Yasara and Uniprot that provided an accessible resource of protein sequence and functional information (Figure 2).
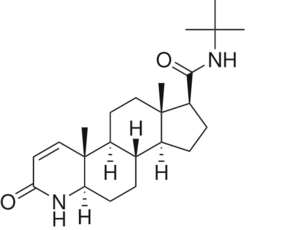
Fig. 1. Structure of Finasteride.
Fig. 1The interaction between 5β-reductase (green) and Finasteride (gray) and NADP (blue). Two Tyrosine (58 and 132 in yellow), two Tryptophan (89 and 230 in red) and Glutamic acid (120 in orange) residues in 5β-reductase are interacting with Finasteride. While Glutamine (193 in light blue) and aspartic acid (53 in purple) residues in 5β-reductase are interacting with NADP.
Mechanism
Finasteride is a 5α-reductase inhibitor. There are three isoforms of 5α-reductase, types I, II, and III. While the drug has a higher affinity for the type II enzyme, it also inhibits the function of the type I and no affinity for type III.[6]The enzyme 5α-reductase turns Finasteride into dihydrofinasteride through an enzyme bound, NADP-dihydrofinasteride adduct.. However, Finasteride , having a high affinity for 5α-reductase, covalently binds to the enzyme as a Michael acceptor, through a functionally irreversible reaction which essentially halts the chemical process before the NADP-Dihydrofinasteride complex can be reduced to Dihydrofinasteride because of a change in the carbanion position in respect to its placement on the similar enolate intermediate created in the reaction where testosterone is converted into DHT by 5α-reductase.(figure 3) However, the NADP-dihydrofinasteride complex does eventually break down with a half life of about 1 month at 37˚C., which is why patients must continue taking the drug.[7]
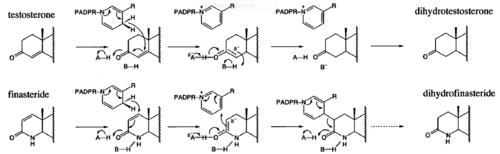
Fig. 3. Proposed mechanism of inhibition by Bull et. al. Image shows how the inhibition of 5alpha-reductase is achieved as Finasteride is used as a substrate to create an enolate intermediate, similar to the one made during the reduction of testosterone. However, the complex created does not allow for the proton transfer needed to complete the reduction of NADP-Dihydrofinasteride to Finasteride, because of the change in the carbanion position. PADPR= phosphoadenosine diphosphoribose
[7] -
Medical Uses
Benign Prostatic Hyperplasia (BPH)
Aromatase and 5α-reductase is responsible for converting androgen hormones into estrogen and dihydrotestosterone (DHT). This chemical process of androgen hormones leads to a decrease in testosterone, but raises levels of DHT and estrogen [8]. Estrogen is a key role in cells proliferating in the prostate and DHT is an anabolic hormone much more potent (dissociated from the androgen receptor slowly) than testosterone that when combined, causes a synergy to induce BPH [9]. The enzyme 5α-reductase is responsible for the synthesis of DHT in the prostate from circulating testosterone. 5α-reductase is located in the stromal cells, which is the main site for the synthesis of DHT, but it can also diffuse into epithelial cells close-by. In both stromal and epithelial cells, DHT binds to nuclear androgen receptors and signals for transcription for cell growth. Finasteride is used to inhibit the 5α-reductase enzyme, which blocks the conversion of testosterone and inhibits the production of DHT, reducing prostate volume and BPH symptoms (urinating complication). Using finasteride could increase the risk for erectile dysfunction, decrease libido, and ejaulation disorder due to 5α-reductase being inhibited.
Prostate Cancer
Prostate cancer is an androgen dependant adenocarcinoma. The FDA has not approved finasteride as a treatment for prostate cancer. A warning was added, 5α-reductase inhibitors concerning an increased risk of high-grade prostate cancer, as the treatment of BPH lowers PSA (prostate-specific antigen), which could mask that prostate cancer is present.
Androgenetic Alopecia (AGA)
Androgenetic alopecia (AGA) is an androgen-dependent, progressively thinning of the scalp hair. AGA is also referred to as male-pattern hair loss or baldness (MPB) and female-pattern hair loss in women. It is characterized by progressive shortening of the duration of anagen with successive hair cycles, leading to decreased numbers of hair in anagen at any given time, and aggressive follicular miniaturization with conversion of terminal to vellus-like follicles [4] The reason for the use of finasteride to treat AGA in men is based on the absence of AGA in men with congenital deficiency of type 2 5α-reductase, and the presence of increased 5α-reductase activity and DHT levels in balding scalp [5]. This condition is caused by a build up of DHT in tissues, which in the case of MPB, is the scalp. The build up of DHT causes androgen-dependent miniaturization of scalp hair follicles. Testosterone is the primary androgen in the body, but to be maximally active in scalp hair follicles it must be converted to dihydrotestosterone (DHT) by the enzyme 5α-reductase. Inhibition of the enzyme with Finasteride has been shown to reduce both serum and scalp skin DHT levels in balding men. In some patients, hair regrowth can occur.[10] Side effects from Finasteride include but are not limited to, decreased sexual ability and desire. [11]