Electron density maps
From Proteopedia
(→2Fo-Fc Map) |
(→Fo-Fc Difference Map) |
||
| Line 40: | Line 40: | ||
===Fo-Fc Difference Map=== | ===Fo-Fc Difference Map=== | ||
| - | Fo-Fc is a "difference map". It shows where the experimental density and the atomic model disagree. If the atomic model fitted the experimental density perfectly, the difference map would have no densities. Typically, it has negative densities (atoms in the model where there is no electron density, isomesh conventionally colored red) and positive densities (electron density where there is no atom in the model, isomesh typically colored blue or green). The isomesh typically represents the boundaries at ±3 sigma, namely, regions with a substantial level of disagreement. | + | Fo-Fc is a "difference map". It shows where the experimental density and the atomic model disagree<ref name="silvaggi" />. If the atomic model fitted the experimental density perfectly, the difference map would have no densities. Typically, it has negative densities (atoms in the model where there is no electron density, isomesh conventionally colored red) and positive densities (electron density where there is no atom in the model, isomesh typically colored blue or green). The isomesh typically represents the boundaries at ±3 sigma, namely, regions with a substantial level of disagreement. |
==Visualizing Electron Density Maps== | ==Visualizing Electron Density Maps== | ||
Revision as of 20:17, 25 July 2021
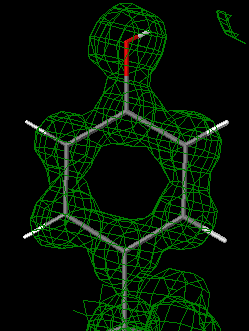
88% of the macromolecular structures available from the Protein Data Bank (PDB) were determined by X-ray crystallography (as of July, 2021). The direct results of crystallographic experiments are electron density maps. Examining the correspondence between the electron density map and the published molecular model reveals regions of uncertainty in the model.
Contents |
Crystallography Produces Electron Density Maps
An X-ray crystallographic experiment produces an electron density map for the average unit cell of the protein crystal. The amino acid (or nucleotide) sequence of the crystallized polymer(s) is known in advance. The crystallographer fits the atoms of the known molecules into the electron density map, and refines the model and map to the limits of the resolution of the crystal (which is limited by the level of order or disorder in the crystal). The crystallographer then deposits a model of the asymmetric unit of the crystal in the PDB, along with the experimental diffraction data (amplitudes and widths of the X-ray reflection spots, or "structure factors") from which the electron density map can be reconstructed. Electron density maps are available for most PDB files from PDBe: at the page titled with the entry ID (4 characters), click on Downloads and look for EDS map and EDS difference map.
Why Look At Electron Density Maps?
Examining the correspondence between the published model PDB file and the electron density map (EDM) provides much clearer insight into the uncertainties in the model than does merely examining the model itself[1] (see also Quality assessment for molecular models). In addition to examining the entire map (2Fo-Fc) it is revealing to examine the difference map (Fo-Fc), which shows where the model fails to account for the map.
2Fo-Fc Map
2Fo-Fc is two times the observed (experimental) density minus the density calculated from the current atomic model. It shows how well the observed density fits around the atomic model[1]. Likely errors are represented by (i) substantial density containing no atom, or (ii) atoms with little or no density. The isomesh typically represents the boundary at 1 sigma.
Isomesh
An isomesh is an isosurface represented as a mesh. An example is in the figure at the upper right of this page. An isosurface is a surface placed at a specific value of a continuous parameter, so that it represents the boundary between higher and lower values of that parameter. Perhaps a geographic map is a more familiar example, where each isoline is a contour line joining positions with the same elevation. In an electron density map, an isomesh joins positions with the same electron density.
Electron density values fill 3-dimensional space of the map (see this interactive visualization). The standard deviation of these values, sigma, is used as a unit of electron density.
Temperature
The agreement between observed electron density and an atom in the model is quantitated in the PDB file as the atom's temperature (also called "B factor"). Thus by coloring the atomic model by temperature, you can see where the electron density fits well vs. poorly, without actually displaying the electron density. In FirstGlance, in the Tools tab, "Local Uncertainty" colors by temperature.
Fo-Fc Difference Map
Fo-Fc is a "difference map". It shows where the experimental density and the atomic model disagree[1]. If the atomic model fitted the experimental density perfectly, the difference map would have no densities. Typically, it has negative densities (atoms in the model where there is no electron density, isomesh conventionally colored red) and positive densities (electron density where there is no atom in the model, isomesh typically colored blue or green). The isomesh typically represents the boundaries at ±3 sigma, namely, regions with a substantial level of disagreement.
Visualizing Electron Density Maps
Crystallographers generally use "heavy duty" visualization and modeling software such as Coot or PyMOL, which require considerable practice to use effectively. Jmol first became capable of displaying electron density maps in January, 2010. Being able to display EDM's in Jmol opens the door to examining EDMs effectively in a web browser, with a user interface (yet to be developed) that requires no specialized software knowledge.
Examples
In Proteopedia
- User:Karsten Theis/Electron density explains and illustrates how to display the electron density map for any selected portion of a crystallographic structure, at various sigma levels.
- Some of the green links in Garman lab: Interconversion of lysosomal enzyme specificities show electron density maps for ligands.
Outside of Proteopedia
- Electron Density: Cloud vs. Isomesh "Map" shows a "raw" electron density map with buttons to hide densities below various sigma "noise" levels. It also shows the isomesh at 1.0 sigma, and the atomic model fitted to the isomesh.
- Electron Density Maps shows the map for bacterial flagellin, resolution 2.0 Å, and shows how the temperature/B-factor relates to the map in areas of low and high temperature.
See Also
Within Proteopedia
- Hydrogen in macromolecular models shows an example of hydrogens visible in an electron density map at 0.69 Å resolution.
- X-ray crystallography
- Resolution
Outside of Proteopedia
References
- ↑ 1.0 1.1 1.2 Lamb AL, Kappock TJ, Silvaggi NR. You are lost without a map: Navigating the sea of protein structures. Biochim Biophys Acta. 2015 Apr;1854(4):258-68. doi: 10.1016/j.bbapap.2014.12.021., Epub 2014 Dec 29. PMID:25554228 doi:http://dx.doi.org/10.1016/j.bbapap.2014.12.021
